*Jarosław Kozakowski, Piotr Dudek
Impairment of male fertility – a consequential problem of our time. The impact of obesity and related metabolic disorders
Zaburzenia płodności u mężczyzn – ważki problem naszych czasów. Wpływ otyłości i związanych z nią zaburzeń metabolicznych
Department of Endocrinology, Centre of Postgraduate Medical Education, Bielański Hospital, Warsaw
Head of Department: Professor Wojciech Zgliczyński, MD, PhD
Streszczenie
Niepłodność, jeden z problemów naszych czasów o narastającym znaczeniu, w dużym odsetku przypadków dotyka także mężczyzn. Wśród wielu czynników branych pod uwagę jako przyczyny tego zjawiska ważna rola przypada otyłości i związanym z nią zaburzeniom metabolicznym. Rozpatruje się wiele mechanizmów, poprzez które nadmierna masa ciała i związane z nią choroby (cukrzyca typu 2, dyslipidemia) negatywnie wpływają na zdolność mężczyzn do rozrodu, zarówno spontanicznego, jak i wspomaganego. W pracy omówiony jest wpływ otyłości i cukrzycy typu 2 na kontrolę hormonalną spermatogenezy oraz bezpośrednie działanie tych czynników na proces produkcji plemników, w wyniku czego u mężczyzn dochodzi do postępującego pogarszania się jakości nasienia i uszkodzenia materiału genetycznego (DNA) komórek zarodkowych. Wskazuje się także na niekorzystną rolę otyłości i cukrzycy przyczyniających się do rozwoju zmian naczyniowych, czego efektem są zaburzenia potencji. Podwyższony wskaźnik masy ciała (BMI), insulinooporność i cukrzyca wpływają także niekorzystnie na procesy dziedziczenia, czego skutkiem jest zmniejszenie możliwości reprodukcyjnych w drugim, a nawet trzecim pokoleniu potomków ojców otyłych.
Rozumienie wpływu otyłości – prawdziwej epidemii XXI wieku, na płodność jest podstawowym warunkiem podjęcia skutecznego przeciwdziała tym niepokojącym zjawiskom.
Summary
Infertility, one of the consequential problems of our time in a great percentage affects men. Among the many factors which are taken into account as the causes of this phenomenon some are attributable to obesity and related metabolic disorders. Quite a few mechanisms by which excess body weight and related diseases (type 2 diabetes, dyslipidaemia) negatively affect the men ability to reproduction, both spontaneous and assisted, are considered. In this review the impact of obesity and type 2 diabetes on the hormonal control of spermatogenesis and the direct effects of these factors on the process of sperm production are discussed. Also the observed result – the progressive deterioration of the semen quality and damage to the genetic material (DNA) of germ cells are elaborated. Moreover, unfavorable role of obesity and diabetes in the development of vascular lesions, resulting in a various degree erectile dysfunctions is briefly presented. Higher body mass index (BMI), insulin resistance and diabetes also affect adversely the inheritance process. In a consequence a decreased reproductive ability in the second and even third generation descendants of the obese fathers is observed.
Understanding the impact of obesity – a real epidemic of the 21st century on fertility is of crucial importance for undertaking effective counteracts to this disturbing phenomena.

Introduction and epidemiology
Impairment of fertility is a great problem of couples nowadays. It is estimated that even 15% of people in the reproductive age in the developed countries may be infertile (1). Epidemiological and clinical data show that every seventh couple in the Western world is infertile currently, and in 40% it results from a “problem” of the man (2). For example, it has been shown in the USA that fertility rates (births per 1000 men) decreased from 57.0 in 1980 to 45.8 in 2013 (3). Similar data comes from Europe. It was found in Denmark that the number of newborns per man at the 45 years of age declined in years 1990-2005 from 1.9 to 1.7 (4).
Sub- or total infertility is a result of several genetic and non-genetic factors. As it has been observed in the numerous cohort studies, one of the more important among these factors is obesity. Data from The Danish National Birth Cohort Study, published in the 2007 year obtained on the basis of the analysis of 47 835 pairs showed that the time to pregnancy (TTP) over 12 months happens much more often when the men has a body mass index (BMI) over 30 kg/m2 compared to men without obesity: AOR (adjusted odds ratio) for obesity in men is 1.53 (95% CI 1.32-1.77). If excessive weight occurs either in female the AOR rises to 2.75 (95% CI 2.27-3.30) (5). Also The Norwegian Mother and Child Cohort Study, involving 26 303 couples has shown that TTP longer than 12 months is observed more often when a man is overweight (BMI > 27 kg/m2). AOR in such situation is 1.20 (1.04-1.38). When a man’s BMI rises above 30 kg/m2 AOR increases to 1.36 (1.13-1.63) (6). The impact of obesity on fertility must be therefore regarded as an important social problem, especially when it is well known that since 1980 the number of obese people all over the world has doubled and now 65% of people live in countries in which excessive body weight is a larger health problem than malnutrition. It is calculated that the health costs of obesity in developed countries reaches approximately £ 2 billion per year (7). Concomitantly, in the last 50 years a continuous decline in fertility has been observed, parallel to the growth of the degree of obesity (8). Male infertility is largely associated with the deterioration of the semen quality. A direct correlation between BMI and a decline in the number and motility of sperm was proved (9). Already overweight, and to a greater extent obesity increase the risk of damage of germ cell’s DNA (10). Moreover, it is well known that excessive body weight affects the sexual possibilities. Erectile dysfunction of a various degree have been found nearly in 96% of the 256 men with metabolic syndrome (11).
Mechanisms of obesity-related male fertility impairment
Considering the factors contributing to the deterioration of fertility in obese men it should be mentioned (fig. 1):
– hypogonadotropic hypogonadism,
– increased aromatization of testosterone to estrogens,
– low sex hormone binding globulin (SHBG) serum levels,
– the deterioration of the quality of semen: reduction of the number of sperm and damage of their DNA,
– peripheral vascular disease leading to erectile dysfunction,
– remote impact of first and perhaps even second generation ancestors obesity (12, 13).
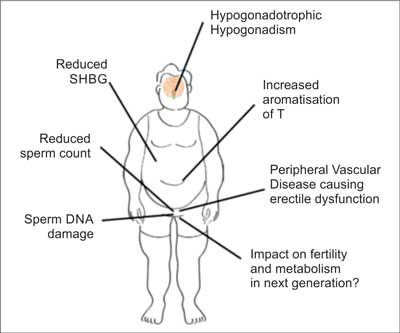
Fig. 1. Factors associated with obesity that affect male fertility (according to (12))
Impact of obesity on the hormonal regulation of reproduction processes in men
Spermatogenesis and sexual ability in men are strictly regulated by the hypothalamic-pituitary-gonadal axis hormones. In obesity which can be treated as a chronic inflammation state this control is disturbed. Excessively expanded and functionally changed adipose tissue becomes a source of many cytokines (adipokines). Some of them, i.e. tumor necrosis factor alpha (TNFα), interleukin 6 (Il-6) as well as several other factors inhibit the production of kisspeptin, a key protein in the mechanism of gonadotropin-releasing hormone (GnRH) secretion in the hypothalamus (14). This effect of adipokines activity is exacerbated by the estrogens. Higher levels of these hormones is a result of the increased aromatization from androgens in large amounts of fat tissue. It has been proved that estrogens, especially in low SHBG conditions actively inhibit the expression of kisspeptin (10). Leptin is an another factor contributing to low kisspeptin production. Although physiologically leptin stimulates GnRH secretion, in case of obesity leptin resistance develops with subsequent inhibition of gonadotropin-releasing hormone synthesis (15). It seems, that kisspeptin expression can be considered as the process that integrates the impact of obesity, testosterone deficiency and the effects of external factors on the hypothalamic-pituitary-gonadal axis. The corollary of the incorrect GnRH secretion is the hypogonadotropic hypogonadism with subsequent testosterone deficiency. In addition, there is a peripheral mechanism that contributes to low testosterone production. This mechanism is again associated with leptin, which blocks the effect of luteinizing hormone (LH) on the Leydig cells and on this way enhancing deficit of androgens (15). In turn, the reduction in the follicle stimulating hormone (FSH) secretion leads to decline in production of inhibin B in Sertoli cells. Inhibin B in normal state stimulates Leydig cells to testosterone secretion and is considered as an indicator of normal spermatogenesis. Its deficiency in obesity may be used as a marker of dysfunction of the seminiferous tubules and reduced number of Sertoli cells.
Impact of obesity on sperm quality
Male infertility is finally a result of abnormal spermatogenesis. The formation of sperm cells in the seminiferous tubules is a complex process regulated in auto-, para-, and endocrine manner. The key hormone in this process is testosterone, which affects the Sertoli and the epithelial cells of the seminiferous tubules. The Sertoli cells provide nutrients and support, being the only somatic cells in direct contact with developing germ cells. They also produce androgen-binding protein (ABP), which contributes towards a higher concentration of testosterone in testes. Moreover, the Sertoli cells secrete many other active biological substances, such as growth factors (TGFα, TGFβ, IGF) which regulate the process of spermatogenesis. In the early stages of prenatal life these cells synthesize anti-müllerian hormone (AMH), which inhibits the development of müllerian ducts exerting influence on the determination of sex. Testosterone also has an effect on other organs important for reproduction – seminal vesicles and tissues associated with erection.
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
29 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
69 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
129 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 78 zł
Piśmiennictwo
1. Homan GF, Davies M, Norman R: The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Hum Reprod Update 2007; 13(3): 209-223.
2. Fritz MA, Speroff L: Clinical Gynecologic Endocrinology and Infertility. 8th ed. Lippincott Williams and Wilkins, Philadelphia 2010: 1137, 1249.
3. Martin JA, Hamilton BE, Osterman MJ et al.: Births: final data for 2013. Natl Vital Stat Rep 2015; 64(1): 1-65.
4. Priskorn L, Holmboe SA, Jacobsen R et al.: Increasing trends in childlessness in recent birth cohorts – a registry-based study of the total Danish male population born from 1945 to 1980. Int J Androl 2012; 35(3): 449-455.
5. Ramlau-Hansen CH, Thulstrup AM, Nohr EA et al.: Subfecundity in overweight and obese couples. Hum Reprod 2007; 22(6): 1634-1637.
6. Nguyen RH, Wilcox AJ, Skjaerven R, Baird DD: Men’s body mass index and infertility. Hum Reprod 2007; 22(9): 2488-2493.
7. Ng M, Fleming T, Robinson M et al.: Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384 (9945): 766-781.
8. Sunderam S, Chang J, Flowers L et al.; Centers for Disease Control and Prevention (CDC): Assisted reproductive technology surveillance-United States, 2006. MMWR Surveill Summ 2009; 58(5): 1-25.
9. Eisenberg ML, Kim S, Chen Z et al.: The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod 2014; 29(2): 193-200.
10. Chavarro JE, Toth TL, Wright DL et al.: Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril 2010; 93(7): 2222-2231.
11. Corona G, Mannucci E, Schulman C et al.: Psychobiologic correlates of the metabolic syndrome and associated sexual dysfunction. Eur Urol 2006; 50(3): 595-604.
12. Chambers TJ, Richard RA: The impact of obesity on male fertility. Hormones 2015; 14(4): 563-568.
13. Fullston T, Palmer NO, Owens JA et al.: Diet-induced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum Reprod 2012; 27(5): 1391-1400.
14. Svartberg J, von Mühlen D, Sundsfjord J, Jorde R: Waist circumference and testosterone levels in community dwelling men. The Tromsø study. Eur J Epidemiol 2004; 19(7): 657-663.
15. Isidori AM, Caprio M, Strollo F et al.: Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999; 84(10): 3673-3680.
16. Ventimiglia E, Capogrosso P, Boeri L et al.: Infertility as a proxy of general male health: results of a cross-sectional survey. Fertil Steril 2015; 104(1): 48-55.
17. Hinz S, Rais-Bahrami S, Kempkensteffen C et al.: Effect of obesity on sex hormone levels, antisperm antibodies, and fertility after vasectomy reversal. Urology 2010; 76(4): 851-856.
18. Bakos HW, Henshaw RC, Mitchell M, Lane M: Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil Steril 2011; 95(5): 1700-1704.
19. Keltz J, Zapantis A, Jindal SK et al.: Overweight men: clinical pregnancy after ART is decreased in IVF but not in ICSI cycles. J Assist Reprod Genet 2010; 27(9-10): 539-544.
20. Bakos HW, Mitchell M, Setchell BP, Lane M: The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl 2011; 34(5 Pt 1): 402-410.
21. Fariello RM, Pariz JR, Spaine DM et al.: Association between obesity and alteration of sperm DNA integrity and mitochondrial activity. BJU Int 2012; 110(6): 863-867.
22. La Vignera S, Condorelli RA, Vicari E, Calogero AE: Negative effect of increased body weight on sperm conventional and nonconventional flow cytometric sperm parameters. J Androl 2012; 33(1): 53-58.
23. Ishikawa T, Fujioka H, Ishimura T et al.: Expression of leptin and leptin receptor in the testis of fertile and infertile patients. Andrologia 2007; 39(1): 22-27.
24. Daxinger L, Whitelaw E: Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet 2012; 13(3): 153-162.
25. Palmer NO, Bakos HW, Fullston T, Lane M: Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012; 2(4): 253-263.
26. El Hajj N, Zechner U, Schneider E et al.: Methylation status of imprinted genes and repetitive elements in sperm DNA from infertile males. Sex Dev 2011; 5(2): 60-69.
27. http://www.mz.gov.pl/aktualnosci/who-oglasza-nowe-dane-o-cukrzycy-na-swiecie/.
28. Bener A, Al-Ansari AA, Zirie M, Al-Hamaq AO: Is male fertility associated with type 2 diabetes mellitus? Int Urol Nephrol 2009; 41(4): 777-784.
29. Melman A, Mason B, Leung AC, Disanto ME: Male Sexual Dysfunction in Diabetes Mellitus. [In:] Poretsky L (eds.): Principles of Diabetes Mellitus. Springer 2016: 401-417.
30. Morano S: Pathophysiology of diabetic sexual dysfunction. J Endocrinol Invest 2003; 26 (3 suppl.): 65-69.
31. Rashid K, Sil PC: Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and endoplasmic reticulum-dependent apoptotic death. Biochim Biophys Acta 2015; 1852(1): 70-82.
32. Shi GJ, Li ZM, Zheng J et al.: Diabetes associated with male reproductive system damages: Onset of presentation, pathophysiological mechanisms and drug intervention. Biomed Pharmacother 2017; 90: 562-574.
33. Torchinsky A, Gongadze M, Orenstein H et al.: TNF-alpha acts to prevent occurrence of malformed fetuses in diabetic mice. Diabetologia 2004; 47(1): 132-139.
34. Kumar TR, Doreswamy K, Shrilatha B, Muralidh: Oxidative stress associated DNA damage in testis of mice: induction of abnormal sperms and effects on fertility. Mutat Res 2002; 513(1-2): 103-111.

