© Borgis - New Medicine 1/2001, s. 16-21
Zofia Rajtar-Leontiew, Elzbieta Lipska
Intracranial haemorrhages in the newborn
Department of Neonatal Pathology The Medical University of Warsaw
Head of the Department: Zofia Rajtar-Leontiew MD, PhD
Summary
The author presents the problem of intracranial haemorrhage, its different types, diagnosis, complications and prognosis in the neonatal period.
Intracranial haemorrhage has been and still is a frequent perinatal complication (1, 3). It is associated with epidural, subdural, subarachnoid, periventricular and ventricular spaces as well as the cerebral tissue, but rarely the cerebellum (fig. 1, 2, 3, 4, 5, 6) (4, 5, 8). It is primarily found in preterm infants (2). However, it may also occur in full-term newborn, mainly those who are the products of pathological, traumatic labour. Haemorrhage may occasionally be accompanied by fractures to the cranial bones. A spontaneous labour does not necessarily exclude a fracture and/or haemorrhage into the central nervous system (CNS) (3).
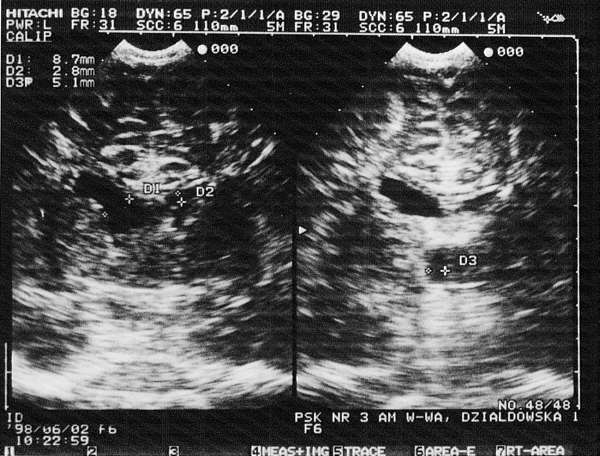
Fig. 1. Complication after an intraventricular haemorrhage: bleeding in the neonatal period (evidently asymmetrical lateral ventricles). Visible significant dilatation and oval contour of the anterior horn on the right.
Haemorrhage into the newborn´s CNS in mainly due to a perinatal head injury, cerebral hypoxia, various congenital defects, hypoplasia of the cerebral blood vessels, their immaturity, and a large difference between intra and extrauterine pressures. In all cases haemorrhage may result from, or be aggravated by, coagulation disorders of different aetiology and pathogenesis (4, 5, 8, 9).
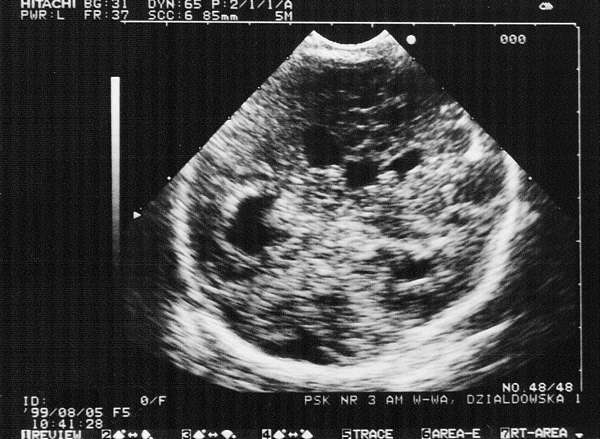
Fig. 2. Bilateral intraventricular haemorrhage: enlarged lateral ventricles with oval contours. Macrogranular choroid vascular plexuses show a large textured cohesion. Visible enlarged third ventricle with a diameter of 7-8 mm.
The best known and researched are peri- and intraventricular haemorrhages, which is largely due to early and repeat USG examination, widely used in our department since 1980, and introduced into our clinical practice by 1962 (6).
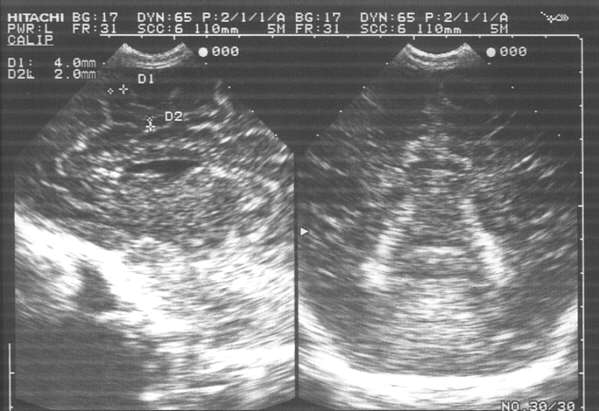
Fig. 3. A healthy, full-term infant with normal body weight. Symmetrical brain structures. Vascular plexuses of lateral ventricles shows a `loose´ texture (coronal plane). A small amount of cerebrospinal fluid present (sagittal plane).
The least frequent in the newborn are extradural haematomas, since at that age their development is prevented by the fusion of the dura matter and the cranial bones. Extradural haemorrhage is caused by fractured cranial bones and a ruptured middle meningeal artery, which, usually in a few hours, leads to substantial extradural accumulation of blood. Such a condition requires an urgent surgical intervention.
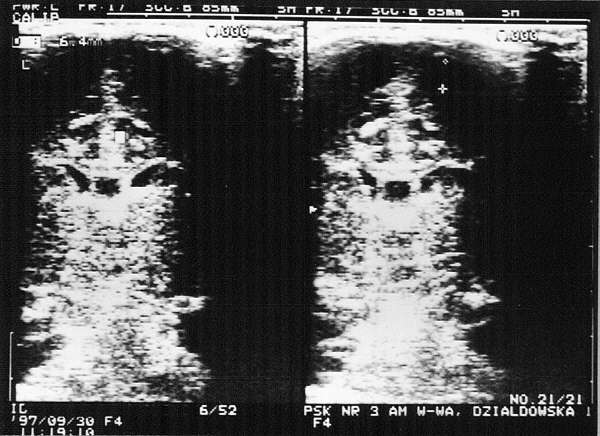
Fig. 4. Subarachnoid haemorrhage: haemorrhagic foci (6-8 mm in diameter) paramedian in the frontoparietal area. Lateral ventricles are normal in size. Visible additional ventricle in the septum pellucidum.
Subdural haemorrhage occurs mainly in full-term infants and is most frequently caused by a rupture of veins at their confluence into the longitudinal cerebral sinus. Injury may occur to the internal cerebral veins, v. of Galen cerebral tentorium or falx, sagittal sinus and/or laternal sinuses. Clinical manifestations accompanying the subdural haematoma depend on the severity of haemorrhage and also on the onset of the condition: acute, subacute or chronic. The criterion we used was the time period from the labour when the condition has its onset and regression, but not its duration. An acute and dramatic development of haematoma occurs after 5 days from injury. The newborn has impaired consciousness, seizures, dyspnoea, opisthotonus, pinpoint pupils, movement of the eyeballs `towards the haemorrhage site´, and papilloedema at the fundi. Frequently, haematoma may lead to coma. The newborn who survives the acute phase of the condition, may, in infancy, develop hydracephalus secondary to a disturbed cerebral flow or absorption of the cerebrospinal fluid.
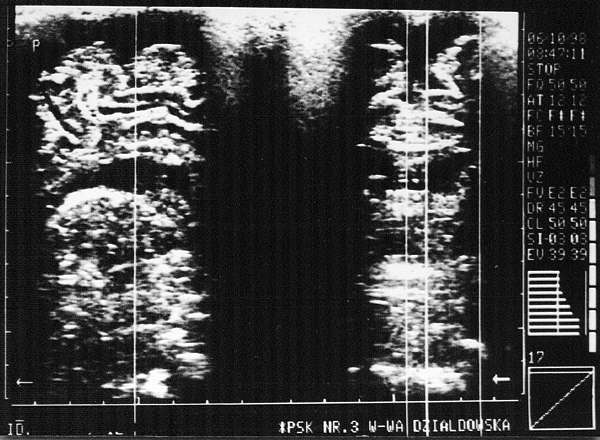
Fig. 5. Subdural haemorrhage. Visible extensive 8-9 mm wide sickle-shaped fluid areas in the apical cerebral vaults. The fluid spaces are also visible in the sagittal plane.
In subacute condition various nonspecific symptoms and signs appear within 6 weeks from the injury, i.e. vomiting, strabismus, a „setting sun sign ”. Later, a chronic haematoma is recognised. It is then very difficult to diagnose considering even a 6-month period from delivery, variability and nonspecificity of clinical manifestations as well as difficulty in its visualisation on the USG examination, even using special technique (frequency transducer Mhz and a distancetip). When the location is very close to the cerebral surface, computer tomography (CT) is the investigation of choice. In such cases translumination with a diaphanoscope may be used as an exploratory examination.
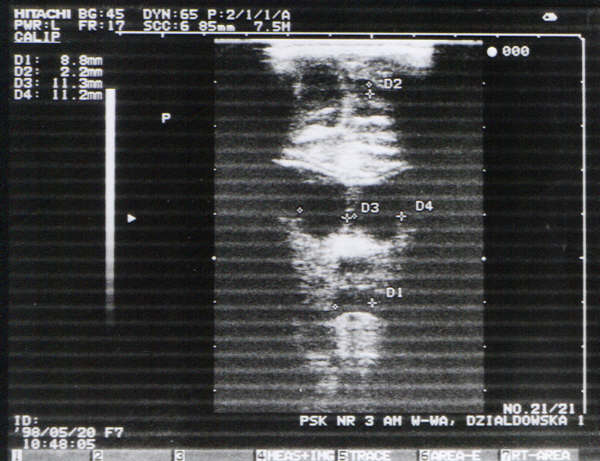
Fig. 6. Haemorrhage into the brain tissue in the diencephalon (coronal plane: enlarged circular lateral, first and third ventricles with a diameter of 9-11 mm). Irregular contour of increased echogenecity between lateral and third ventricles is compatible with the haemorrhage site.
Subarachnoid haemorrhage is very rarely recognised by neonatalogists and has received very little attention in textbooks, although its frequency is estimated at 14%-16% of the total cases of intracranial haemorrhage in the newborn (7, 9). This is due to a number of reasons:
1. subdural haemorrhage is usually asymptomatic;
2. it is not visible on USG examination (with a few exceptions);
3. in the course of the condition, early and late complications occur infrequently and account for less than 10% of the total cases;
4. treatment is medical, and surgical intervention consists of repeat lumbar punctures.
Subdural haemorrhage, or bleeding into the space filled with cerebro-spinal fluid, on the cerebral surface and the spinal cord, usually originates from veins or an arteriovenous involutional anastomosis. The bleeding may be primary, isolated or secondary (accompanying intraventricular, but rarely subdural haemorrhage. The primary subarachnoid haemorrhage occurs mainly in full-term infants and is usually localised in the temporal and occipitaparietal regions, and over the cerebellum. A small, but larger, gradually increasing subarachnoid haemorrhage does not produce any clinical manifestations in the newborn. A larger, or extensively progressing haemorrhage may (usually at two days of age) result in convulsions. In that case the newborn´s condition is usually diagnosed as `good with convulsions´ (1). In massive and rapidly increasing subarachnoid haemorrhage the course of the condition may be severe and the outcome may be fatal. In the most severe cases it is recommended to consider cerebral vascular defects, including congenital haematoma. Secondary subarachnoid haemorrhage usually accompanies subtentorial bleeding, develops simultaneously, and is associated with perinatal cranial fracture. A USG examination in the diagnosis of subarachnoid haemorrhage is useless (7). Haemorrhage into the sylvian sulcus and its dilation as well as ventricular asymmetry may only be indicative of a presumed subarachnoid haemorrhage, and so initiate further more specific diagnostic investigations (9). At that point, it is recommended that assess the cerebrospinal fluid for the presence of erythrocytes and to differentiate between haemorrhagic fluid and simple bleeding occuring on lumbar puncture. If possible, the fluid should be collected into 2-3 tubes, and evaluation should be done of its cleansing, colour, erythrocyte sedimentation rate, their count in 1 ml3, albumin and glucose concentration and possible blood clot formation. Additionally, using accessible techniques, intracranial pressure, ophthalmological examination and EEG should be done. Computer tomography and/or magnetic resonance are helpful in diagnosing not only the type of haemorrhage into the CNS and its effects, but sometimes, from the presence of haemosiderin deposits, it is possible to assess previous bleeding episodes. In subarachnoid haemorrhage 20%-32% of the newborn are diagnosed to have coexistant retinal bleeding. EEG findings may also contribute to the diagnosis and treatment. The tracing may be inactive, hyperactive, periodic paroxysmal with sharp waves, or periodic silence with discharge. An EEG recording inactive for 24 hours is the poorest prognosis. Periventricular, intraventricular, intracerebral and intracerebellar haemorrhage is diagnosed mainly with transoccipital USG examination and the commonest cause is hypoxia of the foetus and/or the newborn. The predisposing factors are hypercapnia, acidosis, acute hypertension, haemostatic disorders, rapid infusion of sodium bicarbonate, pneumothorax, RDS, and congenital heart defects. The less mature the newborn are the more frequently they suffer from haemorrhage to the cerebral ventricules and tissue. Reported figures show an incidence of 40%-50% with less than 32 Hbd and 1500 g, and 80% with less than 1000 g in a group of „live born ” preterm infants. In the first 24 hrs of life, haemorrhagic episodes occurs in 60% of the total cases, at three days it is 85% and by the end of the first week - 95%. Depending on the site and and extent of haemorrhage, the course of the condition may be asymptomatic, moderate, up to severe, even life-threatening clinical manifestations. They include a bulging fontanel, decreased haematocrit value, apnoea, bradycardia, acidosis, convulsions, coma, pinpoint pupils, decreased muscle tone and quadriplegia.
Haemorrhage into the cerebral ventricles and brain tissue may be mistaken for sepsis, meningitis, encephalitis, metabolic disorders, hypoxia or ischaemia. A comparison of clinical diagnoses with the results of USG examinations shows that out of 100 cerebral haemorrhagic episodes suspicion arose in only 60 newborn and, on the average, 25% did not show any lesions, though these had been suspected from the physical findings. In confirmed intraventricular haemorrhagic episodes the cerebrospinal fluid was normal in 20% of patients, and in the remaining patients erythrocytes, leukocytes and increased protein concentration were present. After a few days the CSF became xanthochromic and the glucose level decreased. Therefore, diagnosis, course and prognosis of intracranial haemorrhage cannot be established from clinical manifestations and CSF examination. Assessment of haemorrhage into the ventricles, cerebral and cerebellar tissue, its extent according to Papile´s four-point scale and Monset-Couchard´s modification (introducing 0 for the absence of symptoms and a separate classification for each hemisphere) can be performed and recorded with a repeat USG examination. However, we should not forget that this technique is not reliable in subdural haemorrhage, and it is totally usuless in subarachnoid haemorrhage when blood is mixed with the CSF and flows into the spinal canal.
In such a case, a question arises about what procedure may be helpful in diagnosing intracranial haemorrhage when the USG examination fails to do so, and other imaging examinations of the head are not easily accessible or required transport, which is not unimportant to a neonate´s condition.
Therefore, it seems necessary to monitor the patient, carry out constant measurements of the pulse rate, respiratory rate, and blood pressure, carry out blood examinations, determine the level of bilirubin, assess the muscle tone and sucking ability, perform ophthalmic and repeat neurological examinations, as well as lumbar puncture to evaluate the CSF. In a presumed intracranial haemorrhage, which may be diagnosed only using magnetic resonance or CT, the examination should be done with a simultaneous assessment of the cranial bones. In most cases fractures to the cranial plate are frequently accompanied by intracranial haemorrhage and occasionally subperiosteal haemorrhage which is visible on inspection.
Both linear and focal depressed skull fractures occur most commonly in full-term infants from pathological labour, but also quite frequently in those delivered spontaneously. The fractures are due to a cephalopelvic disproportion a pubic symphysis which fails to dilate or an undiagnosed hyperplastic promontory of the sacral and/or ischial bones. These obstacles result in excessive pressure on the infant´s head during labour, which, with an accompanying difference between intrauterine and external pressures, contributes to bone fractures and/or haemorrhage. Linear skull factures, uncomplicated by haemorrhage, are asymptomatic, usually undetected and do not require treatment. Large depressed skull fractures are corrected surgically in most cases. Diagnosing the condition of a neonate´s cranial bones is not easy. It includes a double-view radiological examination and x-ray films should be assessed by a highly experienced paediatric radiologist.
The incidence of intracranial haemorrhage or accompanying fractures and periosteal haemorrhage has not yet been estimated. Prior to the introduction of the USG, CT and MRI techniques, the data was based solely on postmortem examination of the newborn, whose deaths were due to variety of causes. Nowadays, although the intravital diagnostic procedures are known, they are not effectively used because of insufficient access in many centres. Thus, without well-programmed uniform large-scale multicentre studies of haemorrhage into the central nervous system in the newborn, it will not be possible to fully assess the real extent of the problem for a long time.
Own study
Assessment of intracerebral haemorrhage in the newborn admitted to the Department of Neonatal Patology was carried out in order to:
1. evaluate the scale of the problem;
2. determine a high risk group (from the total number of patients hospitalised at the Department over the last 5 years);
3. attempt to find record which might allow us to suspect other types of haemorrhage than those into the cerebral ventricles. This might help qualify the newborn for less accessible but necessary imaging procedures (CT and/or MRI);
4. assess the frequency of early complications of haemorrhage into the CNS;
5. use the obtained data to try to determine newborn groups for planning further CNS examinations.
The study included 888 neonates and preterm infants admitted over a period of 5 years (from 1 January 1994 to 31 December 1998). In all the patients (treated for different reasons) at least one transfontanelle USG examination had been performed which, in 79 infants (8.9%), showed evidence of haemorrhage into the cerebral ventricles classified from I to IV according do Papile´s criteria. In the newborn with diagnosed haemorrhages, the USG examination of the head was repeated (using accepted standards), and allowed us to observe the disease evolution and to make a detailed descritpion using the scale by Monset-Couchard et al. The largest group of haemorrhagic episodes included those which were finally classified as II and III (total 50--72.1%) (Table 1).
Table 1. Intracranial haemorrhages (IVH) in newborns in the Neonatal Clinic.
| Year | Number | IVH
(%) | Papile scale | Birth weight | Gestational age | Labour | APGAR | ICU | Haemorrhages |
Treated
(total) | IVH | I° | II° | III° | IV° | ELBW
<1000 | VLBW
<1500 | LBW
<2500 | AGA
> 2500 | <
30 | < 34 | < 37 | >
38 | CC | Trauma | Twins | 0-3 | 4-7 | 8-10 |
| 1994 | 193 | 34 | 17.6 | 5 | 14 | 15 | - | 3 | 8 | 10 | 13 | 11 | 3 | 4 | 16 | 8 | 2 | 1 | 4 | 12 | 18 | 9 | 2 |
| 1995 | 182 | 23 | 12.6 | 5 | 11 | 6 | 1 | 2 | 4 | 9 | 8 | 4 | 5 | 5 | 9 | 10 | 1 | 3 | 5 | 8 | 10 | 6 | 3 |
| 1996 | 176 | 9 | 5.3 | 4 | - | 1 | 4 | 1 | 3 | 3 | 2 | 4 | 3 | - | 2 | 5 | - | 4 | 2 | 4 | 3 | 5 | 2 |
| 1997 | 181 | 9 | 5.0 | 2 | 6 | 1 | - | 2 | 1 | 2 | 4 | 3 | 2 | - | 4 | - | 2 | 1 | 2 | 4 | 3 | 1 | 2 |
| 1998 | 161 | 4 | 2.5 | - | 3 | - | 1 | 2 | 1 | - | 1 | 2 | 1 | - | 1 | - | 1 | 1 | 1 | 3 | - | 1 | 1 |
| Total | 888 | 79 | 8.9 | 16 | 34 | 23 | 6 | 10 | 17 | 24 | 28 | 1 | 14 | 9 | 32 | 23 | 6 | 10 | 14 | 31 | 34 | 22 | 10 |
| % | 100 | 100 | - | 20.3 | 43.0 | 29.1 | 7.6 | 12.7 | 21.5 | 30.4 | 35.4 | 30.4 | 17.7 | 11.4 | 40.5 | 29.1 | 7.6 | 12.6 | 17.8 | 29.2 | 43.0 | 27.8 | 12.6 |
Only three neonates had a presumed subarachnoid haemorrhage as assessed from a dilated sylvian sulcus (2 patients) and subcutaneous petechiae on the head, parotid area and neck (1 patients).
Extracranial, subperiosteal haemorrhage was diagnosed in 16 full-term patients (1.8%); 2 patients had intraventricular haemorrhage and in 2 infants radiological examiantion of the skull showed bone fractures.
Out of the 79 neonates with cerebral intraventricular haemorrhage, 51 (64.6%) were preterm infants including 10 (12.7%) with an extremely low birth weight (ELBW), 17 (21.5%) with a very low birth weight (VLBW) and 24 (30.4%) with a low birth weight (LBW). Only 28 newborn (34.5%) had a body weight of over 2500 g and 32 (40.5%) were full-term infants.
Twenty-three (29.1%) infants were delivered by caesarean section, 6 patients (7.6%) had a traumatic birth and 10 were products of twin pregnancies.
Among the infants with haemorrhage into the CNS 14 (17.8%) had a very low Apgar score (0-3), 31 (39.2%) had a low score (4-7) and 34 (43.0%) had a score between 8-10. Twenty-two infants (27.8%) were admitted to the ICU. Thirty-five infants (44.3%) required respirator support for a few days to a few weeks, 18 patients (22.8%) developed RDS I-IV and 9 of those were treated with surfactant. Parenteral nutrition was provided for a few days to several weeks for 31 patients (39.2%) including 4 infants with necrotising enterocolitis (NEC) and 3 infants with presumed NEC. Neurological manifestation were demonstrated by 32 infants (40.5%) including: falccidity, muscular paralysis or increased tone, opisthotonus, spasm, asymmetric reflexes, limb paralysis, tremor, convulsions, lethargy or agitation, nystagmus, a setting sun symptom, and lip smacking. The cerebrospinal fluid was examined in 38 patients (48.1%) and showed abnormalities in 15 infants (19.0%) including 5 infants (6.3%) with diagnosed purulent cerebrospinal meningitis. A quickly progressing hydrocephalus was found in 5 infants (6.3%) and 2 of them underwent a surgical valve implantation. Two infants with hydrocephalus had a coexisting purulent meningo-encephalitis; 1 showed symptoms of brain tissue malacia and 1 developed calcifications. Disorders in the muscle tone were noted in 20 infants (25.3%), impaired hearing in 3 infants and retinopathy (ROP) grade I to IV was found in 9 patients (11.4%). The total number of poorly prognostic factors was noted in 39 infants (49.3%) (Table 2).
Table 2. Other medical problems treated in newborns with IVHs.
|
Year
| Nr of
newborns
with IVH | Asphyxia | Mechanical
ventilation | RDS | Surfactant | Parenteral
feeding | Neurological
symptoms | CSF | Brain damage | Movement
disorders | Hyperbili-
rubinemia | Hearing
abnorm-
alities | ROP | Inborn
and
genetic
defects | NEC | Deaths |
| Analysed | Positive | Meningitis | Hydrocephalus |
| 1994 | 34 | 6 | 13 | 4 | 3 | 12 | 17 | 20 | 5 | 2 | 2 | 14 | 17 | 1 | 3 | 10 | 2 | 4 |
| 1995 | 23 | 5 | 10 | 5 | 2 | 10 | 4 | 8 | 3 | 2 | - | 4 | 11 | - | 1 | 9 | 3 | 3 |
| 1996 | 9 | 6 | 5 | 5 | 4 | 5 | 4 | 3 | 3 | 1 | 2 | 9 | 2 | 2 | 1 | 1 | 1 | - |
| 1997 | 9 | 3 | 4 | 3 | - | 2 | 4 | 4 | 2 | - | - | 5 | 3 | - | 1 | 2 | - | 1 |
| 1998 | 4 | 2 | 3 | 1 | - | 2 | 3 | 3 | 2 | - | 1 | 7 | 2 | - | 3 | - | 1 | - |
| Total | 79 | 22 | 35 | 18 | 9 | 31 | 32 | 38 | 15 | 5 | 5 | 39 | 35 | 3 | 9 | 22 | 7 | 8 |
| % | 100 | 27.8 | 44.3 | 22.8 | 11,4 | 39,2 | 40.5 | 48.1 | 19.0 | 6.3 | 6.3 | 49.4 | 44.3 | 3.8 | 11.4 | 27.8 | 8.9 | 10.1 |
Thirty-five infants (44.3%) with CNS haemorrhage and 16 infants with subperiosteal haemotoma showed an increased level of bilirubin and a slower decrease in its concentration.
Table 3. Cephalhaematomas.
| Year | Number | Hyperbilirubinaemia | Mean weight |
| 1994 | 2 | 2 | 3455 |
| 1995 | 5 | 5 | 3390 |
| 1996 | 3 | 3 | 3600 |
| 1997 | 3 | 3 | 3660 |
| 1998 | 3 | 3 | 2930 |
| | 16 | 16 | 3407 |
Twenty-two infants (27.8%) with CNS haemorrhage had congenital defects and 8 of those had genetic disorders. Eight infants died (10.1%), however, CNS haemorrhage was not the direct cause of death. Seven out of that number were very young preterm infants, 5 had congenital defects, and 4 developed severe sepsis coexistent with NEC in two cases.
On discharge from hospital 59 infants (83.0%) out of 71 survivors were qualified for a further paediatric and specialist follow-up.
Discussion
Haemorrhage into the CNS in the newborn is a significant clinical problem, and also a diagnostic problem despite the wide availability of transfontanelle USG examination. In the present study 888 patients were studied, and out of that number the CNS haemorrhage was diagnosed in 79 (8.9%) infants treated at our Department over a period of 5 years. Preterm infants are the group at the highest risk of developing haemorrhage. The lower their body weight and gestational age, the higher the incidence of haemorrhage. Apart from that, other contributing factors included hypoxia, caesarean section, birth trauma and multiple labour. The extent of haemorrhage into the brain matter, ventricles and tissue, its progress over the subsequent days and weeks of age, complications such as malacia, defects in the brain tissue, calcification and development of hydrocephalus can all be well - visualised and recorded on the transfontanelle USG examination (fig. 1, 2, 3, 4, 5, 6).
Other types of haemorrhage such as extradural, subdural and subarachnoid do not provide a uniform clinical picture, and are not detected on the USG examination, thus escaping diagnosis. Neither does the present paper provide clinical or neurological associations which, in the assessment of the CSF, might lead to a presumption that would render it necessary to perform CT and/or MRI in particular cases. A CNS haemorrhage in the newborn is most frequently associated with other abnormalities. Therefore, it is not possible to isolate the effect and significance of haemorrhage for the final prognosis, particularly when combined with premturity. Not neglecting other causes of hydrocephalus, it seems that the CNS haemorrhage, mainly a massive one, contributes to the development of the complication: in our study it concerned 5 affected infants (6.3%).
Regardless of the question whether poorer development of a newborn or infants is primarily or secondarily dependent on CNS haemorrhage, the fact that as many as 39 newborn (49.4%) were included in the group of fatal prognosis and 83% of the patients were referred for further paediatric and specialist follow-up, should force us to consider the issue and plan future examinations with division into appropriate groups. This would allow researchers to provide a closer association between symptoms and complications exclusively with extravasion into particular spaces within the skull. The present paper should only be considered as a pilot study.
Piśmiennictwo
1. Avery G: Neonatology, Lippincott Company, Philadelphia, London, Mexico City, New York, 1987. 2. Band O, Allest A, et al.: The early diagnosis of periventricular leucomalacia in premature infants with positive rolandic sharp waves on serial electroencephalography. J Pediatrics 1998, 132, 5:813. 3. Fanconi G, Wallgren A: Pediatria, PZWL, Warszawa 1971. 4. Gomella TL: Neonatology, Appleton and Lange, Norwalk 1989. 5. Lozinska D, Twarowska I: Neonatologia, PZWL, Warszawa 1993. 6. Marcinski A: Ultrasonografia pediatryczna, Sanmedia, Warszawa 1994. 7. Pawlowski M, Godula-Stuglik U: Ocena przydatno sci przezciemiaczkowej USG w wykrywaniu uszkodzen OUN u noworodków leczonych w oddziale I.T., Postepy Neonatologii (pod red. Twarowskiej I i Szczapy J), Poznan 1993:198. 8. Schaffer and Avery S: Diseases of the Newborn. Saunders Company, Philad, London, Toronto, Montreal, Sydney, Tokyo 1992 (VI). 9. Zak L, Kornacka M: Krwawienia podpajeczynówkowe u noworodków, Postepy Neonatologii (pod red. Twarowskiej I, Szczapy J), Poznan 1993:129-136.





