© Borgis - New Medicine 1/2001, s. 42-47
Marek Kulus
Bronchial reactivity and initial airway status in asthmatic children
Department of Paediatric Respiratory Diseases and Allergology, Medical University of Warsaw Hospital, Warsaw, Poland
Summary
Bronchial hyper-reactivity (BHR) to various stimuli is a characteristic feature of asthma. However, it is not always possible to perform a bronchial provocation test (BPT) to assess bronchial reactivity. There is no general agreement as to whether the result of a BPT can be predicted on the basis of routine lung tests. The aim of the study was evaluation of the relationship between BPT results and baseline lung function tests, especially assessing the status of small bronchi in children suffering from asthma.
The investigated group comprised 139 children aged 7 to 17 years, with episodic, mild, or moderate asthma. Lung function was assessed on the basis of spirography, flow-volume curve, and airway resistance. Bronchial challenges were carried out with carbachol.
The results showed a good correlation between a BPT result and baseline lung function. Children with impaired initial lung tests had more pronounced bronchial hyper-reactivity. This relationship was closest in the group of children with obstruction of the small bronchi. Analysis of the correlation showed a highly significant relationship between baseline lung function tests and the degree of bronchial reactivity (PC20). The highest significance was observed for MEF50 and MEF25 (p<0.001).
We conclude that small bronchi tests in children with asthma can predict the results of a bronchial challenge with high probability.
Bronchial hyper-reactivity (BHR) to different allergic and non-allergic stimuli is regarded as one of the basic features of asthma. However, it is not always possible to detect BHR in children suffering from asthma. Some data show that it is observed only in about 80% of asthmatic children, and its absence does not exclude asthma. Other authors stress that the use of improper diagnostic tools or misinterpretation indicates an absence of hyper-responsivity in asthma, and that there is no asthma without BHR. Unfortunately BHR is not its exclusive attribute. It is observed in such diseases as cystic fibrosis, atopic rhinitis, chronic lung diseases, congestive heart diseases or even atopic dermatitis. Common viral bronchitis can also induce bronchial hyper-responsivity lasting several weeks. The prevalence of BHR in those diseases is lower, and the course seems to be less severe than in bronchial asthma. Therefore the presence of considerable bronchial hyper-responsivity in patients with ambiguous clinical symptoms of asthma can be decisive in the diagnosis of asthma.
A significant decrease in bronchial obstruction after use of a bronchodilator is also found in bronchial hyperreactivity in asthma. However, in the asymptomatic period when there is no bronchoconstriction the usefulness of bronchial provocation tests (BPT) as a diagnostic tool or for the evaluation of the efficacy of anti-inflammatory treatment is rather questionable. A bronchial challenge requires a proper respiratory laboratory and some experience in the interpretation of BPT results. Sometimes it is impossible to perform the test because of a refusal by the patient or the patient´s parents. Therefore it seems valuable to know if it is possible to predict the results of a bronchial challenge on the basis of routine lung function tests.
The aim of the study was to investigate the correlation between lung function indices, especially describing small bronchi patency, and the results of bronchial provocation tests in asthmatic children.
Material and Methods
Subjects
The investigated group comprised 139 asthmatic children (44 girls and 95 boys) aged 7 to 17 years (mean 9.4 ± 3.5 yrs). The children were patients of our Outpatient Clinic for children with bronchial asthma. All of them were suffering from an episodic, mild, or moderate form of the disease. The diagnosis was established on the basis of the patient´s clinical history, skin prick tests, lung function tests, and immunological investigations.
Method of study
At the moment of the study children were asymptomatic and had been free of acute respiratory tract infections for at least 6 weeks, without any subjective signs of bronchial obstruction, and appeared well on physical examination on the day of the challenge. The course of the disease allowed for a temporary cessation of treatment, which might influence BPT results according to ERS guidelines (26). Baseline lung function tests, including vital capacity (VC), forced expiratory volume in one second (FEV1), maximal expiratory flows for 50% and 25% of forced vital capacity (MEF50, MEF25), and airway resistance (Raw), were performed immediately prior to investigation. We also evaluated indices derived from a flow-volume curve - i.e. mean flow time at 50% of VC (T50), being the result of dividing the half VC by MEF50, and mean flow time at 25% of VC (T25), being the result of dividing the quarter VC by MEF25. The surface under the second half of the flow volume curve (S50) was also calculated. Patients with bronchial obstruction (decreased FEV1%VC below 74%) were not included in the study. A bronchial provocation test was performed next with carbachol. Bronchial challenges were done between 9 and 11 a.m. during the winter months in three consecutive years.
Carbachol challenge
Carbachol chloride solution in buffered saline was prepared immediately before the challenge. An initial inhalation of nebulized, chloride saline preceded each challenge. Subsequently two-fold concentrations starting at 156 mg/ml up to 5mg/ml of carbachol were used during two-minute-long tidal breathing nebulizations (Pari Standard nebulizer). VC, FEV1, MEF50, MEF25, Raw (Body test, Jaeger) and Rrs were measured following each nebulization. Inhalations were provided at 5-minute intervals until the maximal concentration of carbachol was achieved or a 20% fall in FEV1 had occurred. The provocative concentration that induced a 20% fall in FEV1 (PC20) was calculated.
Patients classification
According to the baseline FEV1, MEF50 and MEF25 results, patients were divided in the following manner.
? Group A - with FEV1 higher than predicted for height.
? Group B - with FEV1 equal to or lower than predicted for height.
The division of the children according to their small bronchi test results was done in two ways:
? Group I - with MEF50 or MEF25 higher than predicted for height.
? Group II - with MEF50 or MEF25 equal to or lower than predicted for height and
? Group N - with MEF50 or MEF25 within normal ranges.
? Group O - with MEF50 or MEF25 below normal ranges (value predicted for height less 2 standard deviations) - i.e. a group of children with small bronchi obstruction.
Children were also divided into 5 groups according to their bronchial provocation test results, grading bronchial reactivity. The method used by Woolcock et al. (29) was used with our own modification based on carbachol molar weight.
1. Very severe hyper-reactivity (logPC20 < 4.99).
2. Severe hyper-reactivity (logPC20 between 5.0 and 7.07).
3. Moderate hyper-reactivity (logPC20 between 7.08 and 8.46).
4. Mild hyper-reactivity (logPC20 between 8.47 and 10.37).
5. Normal reactivity (logPC20 > 10.37).
Parental informed consent was obtained before each test. Warsaw Medical School Ethics Committee approved the study protocol.
Statistical analysis
Results of lung function tests were expressed as standard deviation scores (SDS). For standardization, the predicted values for Polish girls and boys were taken from Haluszka and co-workers, while for MEF50 and MEF25 the predicted values were taken from Zapletal et al. (31) and for T50, T25 and S50 the predicted values were from our own laboratory.
Values of PC20 carbachol were logarithmically transformed to normalize their distribution. Differences between groups were calculated with an unpaired Student´s T-test. The relationship between pre-challenge lung function indices and log PC20 was tested by linear regression analysis. The level of statistical significance was taken as a p-value of less than 0.05.
Results
In the investigated group of asthmatic children 34 patients had MEF50 above the average for the population (group I, SDS <=0). Their bronchial reactivity (log PC20=9.1508 ±2.9218) was significantly lower (p<0.001) than the rest of the investigated patients (group II, SDS<0) (log PC20=6.9261 ±2.6217) (Fig. 1a - page 44). Similarly, the group I contained 41 asthmatics with MEF25 above that predicted (SDS <=0), and showed a lower hyper-reactivity (log PC20=8.8591 ±3.2846) (p<0,001) than group II with lower initial MEF25 values (log PC20=6.8897 ±2.4427) (Fig. 1b - page 44).
Thirty-two children had MEF50 below the normal range (value predicted for height less two standard deviations, SDS<-2) - i.e. children with small bronchi obstruction (group O) (log PC20=6.0044 ±1.4442). The remaining subjects totaling 107 patients had MEF50 within normal limits (group N, SDS <=-2) (log PC20=7.9087 ±3.0310). The groups differed at a significance level of p<0.001 (Fig. 2a - page 44). Twenty-three children with MEF25 below the normal range (value predicted for height less 2 standard deviations, SDS<-2) (log PC20=6.1996 ±1.5157) differed at a significance level of p<0,001 from the group of 116 patients with MEF25 within normal limits (group N, SDS <=-2) (log PC20=7.7222 ±2.9967) (Fig. 2b - page 44).
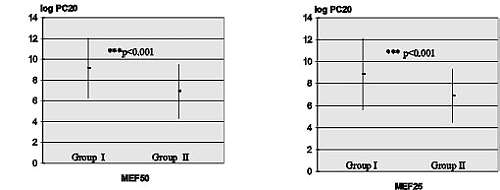
Fig. 1. Comparison of log PC20 between A) group I - with MEF50 higher than predicted for height, and group II - with MEF50 equal or lower than predicted for height, and B) group I - with MEF25 higher than predicted for height, and group II - with MEF25 equal to or lower than predicted for height. ***: significant difference at p<0.001.
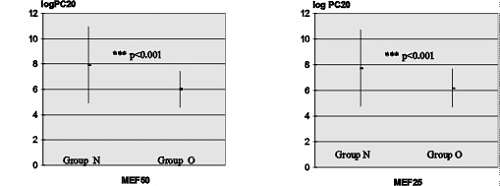
Fig. 2. Comparison of log PC20 between A) group N - with MEF50 within normal limits (SDS <=-2) and group O - with MEF50 below normal range (value predicted for height less 2 standard deviations, SDS<-2) - i.e. with small bronchi obstruction, and B) group N - with MEF25 within normal limits (SDS <=-2) and group O - with MEF25 below normal range (value predicted for height less 2 standard deviations, SDS<-2) - i.e. with small bronchi obstruction. ***: significant difference at p<0.001.
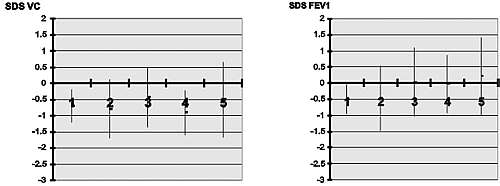
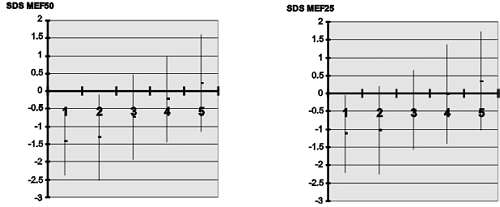
Fig. 3. Baseline lung function test results in children with different bronchial reactivity. 1 - very severe hyper-reactivity (logPC20 < 4.99), 2 - severe hyper-reactivity (logPC20 between 5.0 and 7.07), 3 - moderate hyper-reactivity (logPC20 between 7.08 and 8.46), 4 - mild hyper-reactivity (logPC20 between 8.47 and 10.37), 5 - Normal reactivity (logPC20 > 10.37).
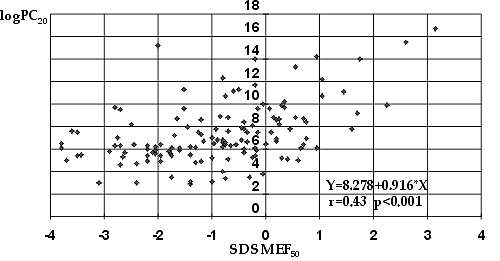
Fig. 4. Relationships between pre-challenge MEF50 and log PC20.
According to the division of patients into 5 groups with different degrees of bronchial reactivity, the largest was group 2, with severe bronchial reactivity (log PC20 between 5.0 and 7.07), containing 63 children. Other groups had a similar number of patients. Comparison of baseline lung function tests in groups showed the worst the initial lung function test result the more pronounced was the bronchial reactivity (Fig. 3 - page 45). Linear regression analysis revealed the closest relationship between pre-challenge MEF50 and log PC20 (R=0.4321 p<0.001) (Fig. 4 - page 45). For most of the investigated lung tests this relationship was also highly statistically significant (Tab. 1).
Table 1. Correlation between baseline lung function tests and log PC20. Fig. 1. Complication after an intraventricular haemorrhage: bleeding in the neonatal period (evidently asymmetrical lateral ventricles). Visible significant dilatation and oval contour of the anterior horn on the right.
| Test | Correlation coefficient | p |
| FEV1%VC | 0.309 | <0.001 |
| MEF50 | 0.432 | <0.001 |
| MEF25 | 0.386 | <0.001 |
| T50 | 0.341 | <0.001 |
| T25 | 0.323 | <0.001 |
Discussion
An enhanced response to stimuli provoking bronchial obstruction is not a constant feature. After viral infections of the respiratory tract bronchial hyper-reactivity may be observed for 4 to 6 weeks, mainly as a result of epithelium damage. In a case of occupational asthma exclusion of the harmful agent from the patient´s environment can gradually diminish the degree of bronchial reactivity, which increases again on returning to the previous conditions. In asthmatic patients, exposure to an injurious environment causes a reaction of the bronchial tree, which is longer and more severe than in normal subjects. A specific bronchial challenge with an allergen can cause bronchial hyper-responsivity lasting from 1 week (5) to 4 months (10). Repeated contact with an allergen induces chronic bronchial hyper-reactivity, probably as the result of the constant stimulation of an inflammatory reaction. Avoidance of the harmful agent is the crucial element of the recovery process and a return of the bronchial reactivity to normal. The classic example is pollen allergy in asthma. Beyond the pollen season patients are not exposed to allergic stimuli and have the most suiTable conditions for recovery from bronchial hyper-responsivity (2). However, such desirable situation does not always occur. Often bronchial hyper-reactivity does not fade out even after years of allergen avoidance.
The cause of bronchial hyper-reactivity is still not completely clear. Amongst many hypotheses proposing an explanation of this phenomenon most data indicates a key role for epithelial injury, allergic inflammation, or structural remodelling of the bronchi (1, 3, 12, 19, 20, 21). The question as to how disturbances in airway geometry can influence bronchial contractility still remains open (1, 25). The diameter of the bronchi changes as an effect of smooth muscle constriction, muscle hypertrophy, mucus overproduction, and swelling of the mucosa (28). According to Laplace´s formula, flow resistance is the reciprocal of the fourth power of the bronchial radius. When the shortening of the bronchial radius remains the same, an agent provoking constriction causes greater changes of resistance in narrower than in wider bronchi. This gave rise to the hypothesis that bronchial reactivity is combined with an initial decrease in bronchial diameter (1). Although the mechanical factor plays an important role in evaluation of bronchial responsiveness, it is not crucial to its development. In several papers differences in bronchial reactivity in patients with the same initial bronchial diameter have been shown. Mentioning bronchial calibre in lung function tests in vivo is of course a considerable simplification, because such tests usually include forced expiratory volume in one second (FEV1), airways resistance (Raw), or conductance (sGaw). They only evaluate airway patency in an indirect way and then mainly of upper passages. There is still not much data about the influence of the peripheral airways on bronchial hyperreactivity (16).
Results of studies investigating the relationship between pre-challenge lung function and airway reactivity often disagree. Benson´s opinion that the outcome of a bronchial challenge depends on pre-challenge bronchial obstruction is generally accepted (1). Almost all studies evaluate airway status on the base of the FEV1 test. An example is given by the studies of Machiels and Ferriere (15) in asthmatics, Taylor et al. (27) in a population of healthy smokers and non-smokers, and Ramsdale at al. (23) in groups of asthmatics and COPD patients. These show a good correlation between FEV1 and the results of bronchial provocation testing. Mullen and co-workers (18) revealed expressed pathomorphologic changes in the bronchi of smokers with bronchial hyper-reactivity. However, Yan et al. (30), Hargreave et al. (11) and Cockroft (4) proved a good correlation between PC20 and FEV1/FVC only in COPD patients, and their absence in asthmatics. Pauwels and Snashall (22) have given an opinion that bronchial obstruction has no implications for bronchial challenge results. Similarly Garcia-Herreros and co-workers (9) disagree with Benson´s findings on the basis of their own studies in asthmatics, COPD and atopic rhinitis patients, where they showed a lack of any relationship between airway resistance or FEV1/FVC and bronchial provocation test results. Eiser et al. (7) also confirmed the lack of such a relationship, investigating specific conductance (sGaw) and challenge results. Moreno et al. (17) in an elegant study showed the mechanisms of bronchial obstruction in vitro. Sheppard (24), on the base of this experimental work, distinguished three basic factors influencing the results of bronchial challenge, which include a decrease of airway diameter, decrease of bronchial smooth muscle preload, and increase of bronchial wall thickness.
The mathematical relationship between bronchial diameter and FEV1, MEF50 and MEF25 is not clear. However there is general agreement that they yield a good picture of the characteristics of airway obstruction. Some indices derived from the flow-volume curve are considered to be an indicator of bronchial obstruction, especially of peripheral airways, and are thus important in the asymptomatic period of asthma. Chronic inflammation in the bronchial tree is often impossible to detect by simple spirography or airway resistance measurements. In children it is relatively uncommon to have a disturbed flow-volume curve caused by other than obstructive factors, as for example impaired lung compliance.
As the population of investigated subjects was relatively homogenous, because all of them were suffering from mild to moderate forms of bronchial asthma, it was interesting to see if there was any reason for discrepancies in bronchial reactivity in this group. The division of children was made on the base of initial lung function tests on two levels: the mean values predicted for height, and the lower normal limit. We found a correlation between baseline lung function tests and bronchial challenge results. Asthmatic children with poorer initial lung tests had significantly higher bronchial reactivity. This correlation was closest in children with disturbed small bronchi. Despite the lack of obstruction in FEV1 (the initial criterion to perform the carbachol challenge), patients with obstruction in the small bronchi (decreased MEF50 and MEF25) had significantly increased bronchial reactivity (p<0.001).
However, investigating differences in initial lung function tests in the groups of children with different degrees of bronchial reactivity, we found that patients with the most severe bronchial hyperreactivity had more obstructed bronchi in baseline lung tests. This part of our study was consistent with the observations of Mullen and co-workers (18) in a group of smoking subjects.
We did not observe bronchial hyperreactivity in 18 of 139 asthmatics (12.9%). The percentage is lower than that found by Kerrebijn (14). However, comparison with that data has to be approached with caution, because the criteria for inclusion of patients into the study and baseline bronchial calibre may have been different.
The correlation analysis showed a highly significant dependency of bronchial challenge results and baseline lung function tests describing bronchial calibre. Models of bronchial challenge that were build for all lung tests proved a good linear correlation between the results and initial lung tests. The best correlation was observed for MEF50 and MEF25. Multiple regression analysis did not improve this relationship.
The results of the study confirm that decreased airway calibre, especially of the small bronchi, has a serious influence on bronchial challenge with carbachol in asthmatic children. It is important to look at more than one lung test to obtain a better picture of lung function. On the basis of only FEV1 or Raw, conclusions about the whole bronchial tree are inaccurate. Therefore, more complex investigations have confirmed the hypothesis that a mechanical factor, indirectly dependent on an allergic inflammatory reaction, has an influence on bronchial reactivity. Investigations of broncho-alveolar lavage in combination with BPT show a similar relationship (8, 13). Detection of bronchial obstruction in the small bronchi allows us to predict the results of BPT with a high probability. It is important to remember this when comparing results between different groups of patients with variable degrees of bronchial obstruction (6). Such a prognostic value of the small bronchi calibre in children is useful when BPT is contra-indicated or impossible to perform for other reasons.
Piśmiennictwo
1. Benson M.K.: Bronchial hyperreactivity. Brit. J. Dis. Chest, 1975, 69, 227. 2. Boulet L.P. et al.: Asthma and increases in nonallergic bronchial responsiveness from seasonal pollen exposure. J. Allergy Clin. Immunol. 1983, 71, 399. 3. Boushey H.A. et al. Bronchial hyper-reactivity. Am. Rev. Respir. Dis. 1980, 121, 389. 4. Cockcroft D.W.: Bronchial inhalation tests. I. Measurement of nonallergic bronchial responsiveness. Ann. Allergy 1985, 55, 527. 5. Cockcroft D.W. et al.: Allergen-induced increase in nonallergic bronchial reactivity. Clin. Allergy 1977, 7, 503. 6. Eiser N.M. et al.: Guidelines for standardization of bronchial challenges with (nonspecific) bronchoconstricting agents. Bull. Europ. Physiopath. Resp. 1983, 19, 495. 7. Eiser N.M. et al.: The effects of atropine on histamine and antigen-induced bronchospasm. Abstracts of the 6th General Meeting of SEPCR, Basel, 1979, 15. 8. Ferguson A.C., Wong F.W.M.: Bronchial hyperresponsiveness in asthmatic children. Chest 1989, 96, 988. 9. Garcia-Herreros P. et al.: Bronchial hyper-reactivity to histamine in allergic and non-allergic asthma, allergic rhinitis, and chronic Ohashi Y. et al.:Airways hyper-responsiveness, increased intracellular spaces of bronchial epithelium and increased infiltration of eosinophils and lymphocytes in bronchial mucosa in asthma. Am.Rev.Respir.Dis.1992, 145,1469-1476. 20. Pauwels R.: Pathogenic mechanisms in childhood asthma. Triangle, 1987, 26(suppl.1), 13. 21. Pauwels R.: The clinical relevance of airway inflammation. Eur. J. Respir. Dis. 1986, 69(suppl.147), 88. 22. Pauwels R., Snashall P.D.: A practical approach to asthma. CBA Publishing Services, Dorking, 1986. 23. Ramsdale E.H. et al.: Differences in responsiveness to hyperventilation and methacholine in asthma and chronic bronchitis. Thorax, 1985, 40, 422. 24. Sheppard D.: Airway hyperresponsiveness. Mechanisms in experimental models. Chest 1989, 96, 1165. 25. Simonsson B.G.: Can we lower airway hyper-responsiveness? Pneum. Pol. 1988, 56, 447. 26. Sterk P.J. et al.: Airway responsiveness. Standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Eur. Respir. J. 1993, 6, 53. 27. Taylor R.G. et al.: Bronchial reactivity to inhaled histamine and annual rate of decline in FEV1 in male smokers and ex-smokers. Thorax, 1985, 40, 9. 28. Thurlbeck W.M.: Structural abnormalities of the peripheral airways. in „Small airways in health and disease. ” Excerpta Medica, Copenhagen 1979, 3. 29. Woodcock A.J. et al.: Does bronchial hyper-responsiveness equate with asthma. Triangle 1988, 27, 67. 30. Yan K.et al.: Prevalence and nature of bronchial hyperresponsiveness in subjects with chronic obstructive pulmonary disease. Am. Rev. Resp. Dis., 1985, 132, 25. 31. Zapletal A. et al.: Lung function in children and adolescents: methods, reference values. Prog. Respir. Res. Krager, Basel, 1987, 22, 114.




