© Borgis - New Medicine 1/2001, s. 62-65
Elzbieta Maciorkowska1, Beata Szynaka2, Maciej Kaczmarski1, Andrzej Kemona2
Platelets and Helicobacter pylori infection
1 IIIrd Department of Paediatrics, Medical Academy of Bialystok, Poland
Head: Professor Maciej Kaczmarski MD, PhD
2 Institute of Pathologic Anatomy, Medical Academy of Bialystok, Poland
Head: Professor Boguslaw Musiatowicz MD, PhD
Summary
The authors evaluated the ultrastructur of the gastric mucosa and fine blood vessels in two girls infected with H. pylori and peripheral blood thrombocytopaenia. Platelet-granulocyte or only platelet aggregates were found in the gastric mucosa of patients under the superficial epithelium in H. pylori accumulation sites, epithelial cell lesions and in the capillary lumen. Platelet aggregates adhered to basement membrane devoid of endothelial cells. Erythrocytes placed loosely or in agglomerations, eosinophil and neutrophil granulocytes, plasmocytes and mast cells were found beyond the vascular bed, most frequently close to vessels in damaged endothelium or with platelet aggregates.
Introduction
Platelets belong to the main components of haemostasis. Their haemostatic function is regulated by numerous binding substances, including their membrane receptors. Thrombin, plasmin, and vessel -derived substances, compounds included in platelet granules such as ADP, thromboxan A2-TXA2, prostoglandyn D2-PGD2, serotonin, von Willebrand-vWF factor and platelet-activating factor PAF condition the normal blood clotting process.
Platelets are also involved in phagocytosis and in natural defence mechanisms. Mustard and Packham were the first to present this phenomenon in 1968 (1). Bacterial phagocytosis with platelets can be carried out via individual platelets (2) or platelet aggregates (3). Platelet activation is possible due to an interaction with immunocompetent cells, IgE, IgG complement components, CRP and lymphokines (4).
Recent studies indicate that platelets are capable of interaction with leukocytes in initiating and maintaining the inflammatory process. Numerous surface platelet proteins and proinflammatory mediators stored in the intracellular aggregates determine the contribution of thrombocytes to such disorders as ARDS (adult respiratory distress syndrome), chronic intestinal inflammation, inflammatory states of intestinal allergic vasculitis, and tuberculosis.
The role of platelets in defence mechanisms in the presence of schistosmosis (5), toxoplasmosis (6) and Giardia intestinalis infection (7) has been described.
Based on increased knowledge of H. pylori, leukocyto-platelet aggregates have been postulated to contribute to gastric lesions in H. pylori infection. Platelet activation caused by this ethiologic factor colonizing the stomach represents an essential vascular component in the pathophysiology of this disease (8).
Apart from the well-defined role of H. pylori as a mediator of gastric mucosa inflammation, duodenal ulcers, and in the development of carcinoma in adults, this bacterium takes a pathogenic part in coronary disease (9). Recent observations support the view that H. pylori may have an influence on the gastric mucosa not only through triggering neutrophilic activation and local cytokine production, but through gastric vascularization as well (8).
In the pathogenesis of H. pylori, both a bacterial strain and the host´s immunological response play an important role. H. pylori may produce substances that can directly destroy epithelial cells, decreasing gastric mucosa resistance (10). This bacterium may indirectly cause gastric lesions via cytokine response stimulation in the mucosa (mainly IL-8) (11).
Additionally, H. pylori extracts contain substances inducing chemotactic activity in neutrophils and monocytes that may increase the surface expression of certain molecules affecting neutrophils in particular, due to inflammatory cell response. Increased permeability, leukocyte migration, albumin transudate, mast cell degranulation and platelet-leukocyte aggregation were present with the inflammatory cell response (11).
The purpose of this study was to evaluate the ultrastructural picture of the gastric mucosa and fine blood vessels in children infected with H. pylori and with peripheral blood thrombocytopaenia.
Methods
Ultrastructural examinations of the gastric mucosa were performed in two girls (B.M. and O.C.) aged 12 and 13 years, frequently hospitalised for thrombocytopaenia in the IIIrd Department of Paediatrics, Medical Academy of Bialystok. Because of dyspeptic symptoms in the clinical picture, both girls underwent upper gastrointestinal tract endoscopy. Gastric mucosa was estimated macroscopicaly and histopathologicaly for H. pylori infection (Sydney System assay). During endoscopy the urease test was applied (CLO-test) and mucus was taken from the duodenum to exclude Giardia lamblia infection.
Additionally, biopsy specimens were taken from the antrum, corpus and duodenum for ultrastructural examination. They were fixed in 3.6% glutaraldehyde at 4o C for 2hrs and postfixed in 2% osmium tetroxide for 1hrs. After dehydration in alcohol, they were washed in propylene oxide and embedded in Epon 812. The ultrathin sections were cut on a Reichert ultramicrotome, doubly stained with uranyl acetate and lead citrate, and examined using an electron microscope EM 900 PC (OPTON West Germany).
Results and discussion
Studies carried out on mice Spanish authors prove that platelet aggregation is the most important characteristic of gastric microvascularization in mice infected with H. pylori (8). This effect seems to be highly specific for H. pylori infection, because animals challenged with other bacteria produced only a few platelet aggregates. Some investigators have proved that both leukocyte rolling in blood vessels and platelete aggregation are P and L-selectin-dependent, since blocking these molecules causes a significant reduction in the flow of rolling leukocytes and in a number of aggregates in gastric microvessels. Platelet aggregates in portal blood in H. pylori-infected mice and the inhibition of aggregation after anti P-selectin (mAb) treatment confirm H. pylori infection activation mediated by platelets (8).
Since PAF, leukotrien, tromboxsan and the products of leukocyte and platelet activation are present in the gastric mucosa of patients infected with H. pylori, it seems reasonable to postulate that leukocyte-platelet aggregates take part in the pathogenesis of the gastric mucosa damage.
Numerous changes in fine blood vessels were observed in the ultrastructural picture of the gastric mucosa in H. pylori infected patients.
The lumen of many mucosal capillaries and vessels was filled with erythrocytes mixed with granulocytes, lymphocytes and platelets. These cells were placed loosely or combined forming aggregates (Photo 1).
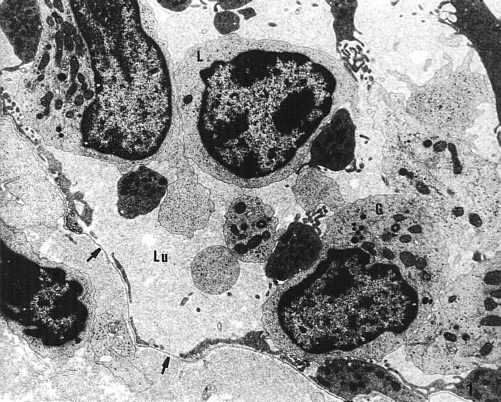
Photo 1. Numerous cells: granulocytes (G) and lymphocytes (L) in the vascular lumen (Lu). Focally damaged endothelial ependyme (?). TEM x 3,000.
Platelet-granulocyte or platelet-only aggregates were found in the gastric mucosa bioptates of our patients under the surface epithelium mainly in gastric pits, H. pylori accumulation sites, epithelial cell lesions and in the capillary lumen (Photo 2).
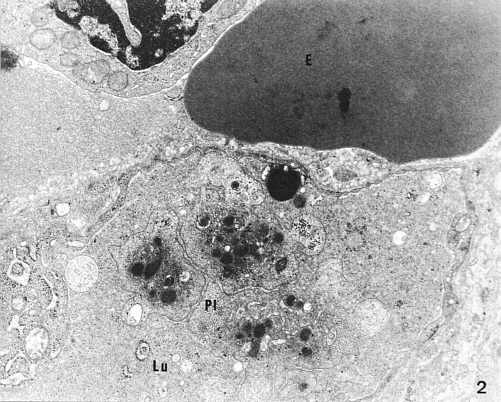
Photo 2. Platelet conglomerate (PL.) in the vascular lumen (Lu). Fragment of erytrocyte seen beyond the vascular bed (E). TEM x 7,000.Fot 2. Platelet conglomerate (PL.) in the vascular lumen (Lu). Fragment of erythrocyte seen beyond the vascular bed (E). TEM x 7,000.
Some features of focal destruction of the endothelial cells in fine blood vessels were observed in the gastric mucosa bioptates of our patients (Photo 1). Numerous erythrocytes, eosinophilic and neutrophilic granulocytes, plasmocytes and mast cells were found beyond the vascular bed, most frequently close to vessels with damaged endothelium (Photo 3).
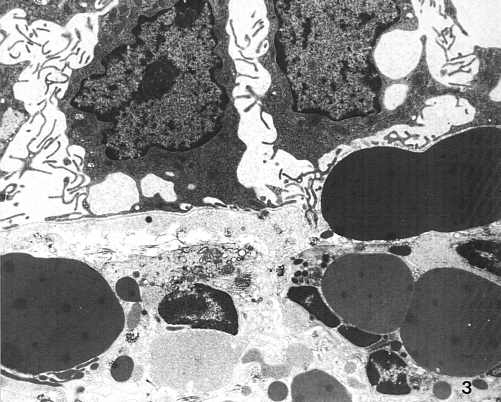
Photo 3. Numerous erythrocytes and inflammatory cells under surface epithelium. TEM x 3,000.
Kalia et al. using an electron microscope confirmed that aggregates in mucosa contained only platelets whereas on mesenteric H. pylori extracts there was induced platelet-leukocyte aggregation. The different responses of these two tissues may be due to differences in adhesive molecule distribution on mesenteric endothelial cells and the gastric mucosa (12).
Platelets themselves are considered to be an important factor in an inflammatory response, a potent source of pro-inflammatory mediators and influencing the activity of other proinflammatory cell activities. Early local response in H. pylori inflammation can become a factor initiating platelet aggregation and leukocyte recruitment. It is quite possible that an obstruction to microvessels caused by clots may lead to focal mucosa disruption and resulting tissue necrosis.
Platelet activation induced by pro-inflammatory mediators (PAF) is closely connected with the synthesis and release of numerous substances contributing to the development of the inflammatory process such, as histamine, serotonin, PDGF (Platelet derived growth factor), TGF, IL-1 leucotriens and prostaglandins.
The role of P-selectin in inflammatory processes has only recently been recognised. The release reaction is manifested in the increase in the surface expression of P-selectin (CD62) and protein GP53 (CD63), which that are markers of trombocyte activation level. The platelet activation mediated by histamin or serotonin causes an increase in P-selectin surface expression due to the translocation of a granules on the surface of the platelet cellular membrane. P-selectin particles existing on the platelet surface (CD62p) and on endothelial cells (CD62E) recognize their specific ligands on myeloidal cells. This recognition results in the appearance of platelet-granulocyte and platelet-monocyte aggregates, due to the combination of lectin structures with ligand glycoproteins (sLeX) in a P-selectin particle. Platelet-granulocyte and platelet-monocyte aggregation stimulates myeloid cells to produce and release toxic supraoxide anionradicals.
Platelet adhesion to the internal surface of damaged endothelium is possible due to functional integrins such as ligand (ICAM-2) present on their surface. The expression of PECAM particles on the platelets enables their diapedesis through vascular endothelium and their aggregation in tissues (8).
Sobhan et al. have estimated the significance and regulatory activity of gastric PAF release in man (13).
PAF as a lipid mediator inducing hypersensivity and many inflammatory reactions is synthesized by many types of human cells including neutrophils, monocytes, macrophages, eosinophils, platelets and vascular endothelial cells. The relationship between the secreted quantity of PAF and severity of gastritis, H. pylori colonization of the gastric mucosa, or gastric acid secretion seems to be essential.
PAF takes part both in the early stage of an inflammatory reaction and in its later development, co-operating with some cytokines. It upregulates IL-1 production through human monocytes, IL-1, TNF- α, IFN- g, which activate PAF syntesis in monocytes.
A number of data describe the modulating influence of PAF on lymphocyte proliferation and IL-2 production. It activates monocytes and macrophages to TNF-α production and facilitates its production by these cells under the influence of other activators such as IFN-g, IL-1, LPS, GM-CSF, and TNF- α.
French authors have analysed PAF gastric secretion and its precursors in response to gastrin, secretin and anti-secretory drugs in patients infected with H. pylori, and in controls. Since PAF is considered to be involved in mucosal damage it was interesting to examine its role in healthy subjects and those infected by H. pylori. The presence of PAF and its precursors in the stomach suggests that PAF may have an ulcerogenic effect on the gastric and oesophageal mucosa. Peptides such as gastrin and secretin modulate gastric PAF (13).
Denizot et al. have proved that H. pylori bacteria produce PAF in vitro. Examinations in H. pylori-infected patients and controls showed no significant differences (14). High quantities of secreted PAF were noted in patients with ulcerous oesophagitis. In this disease gastro-oesophageal reflux, and not over-production of gastric acid, seems to be a mechanism for mucosal damage which could be modulated by PAF and arachidic acid metabolites. These findings support the effect of PAF cellular activity defined as a factor damaging the cell membrane (13).
Conclusions
1. In H. pylori-infected children platelets and platelet-leukocyte aggregates were found in capillaries and extracapillaries. They may be a pathogenetic factor in gastric mucosa damage in children.
2. Focal damage in gastric mucosa vessels and extravascular penetration of blood morphotic elements i.e. erythrocytes, eosinophilic and neutrophilic granulocytes, plasmacytes and mast cells are caused by platelet and platelet - granulocyte aggregations.
3. A decrease in a number of peripheral platelets can be caused by their effect on platelet and platelet-granulocyte aggregations in response to H. pylori infection.
Piśmiennictwo
1. Mustard J.F., Movat H.Z., Glynn M.F.: Platelet phagocytosis. Thromb. Diath. Haemorrh. Suppl. 1965, 17, 271-4. 2. Kemona H., Andrzejewska A., Prokopowicz J. i wsp. Phagocytic activity of human blood platelets examined by electron microscopy. Folia Haematol. 1986, 113, 696-702. 3. Clawson C.C., White J.G.: Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am. J. Pathol. 1971, 65, 367-380. 4. Slezak S., Symer D.E., Shin H.S.: Platelet - mediated cytotoxicity: Role of antibody and C3, and localization of the cytotoxic system in membranes. J. Exp. Med. 1987, 166, 489-505. 5. Polack B., Peyron F., Auriault C.: Platelet cytotoxicity against parasites. Nouv. Rev. Fr. Haematol. 1991, 33, 317-322. 6. Yong E.C., Chi E.Y., Fritsche T.R. Henderson W.R.Jr.: Human platelet- mediated cytotoxicity against Toxoplasma gondii: role of tromboxane. J.Exp. Med. 1991, 173, 65-78. 7. Matowicka-Karna J., Panasiuk A., Olesiewicz B.: Phagocyte activity of platelets in patients infected with Giardia intestinalis. Przegl. Lek. 1996, 53, 785-787. 8. Elizalde J.I., Gómez J., Panes J., i wsp. Platelet activation in Mice and Human helicobacter pylori infection. J. Clin. Invest. 1997, 5, 996-1005. 9. Patel P., Carrington D., Strachan D.P., Leatham E., Goggin P, Northfield T.C., Mendall M.A.: Fibrinogen, a link between chronic infection and coronary heart disease. Lancet 1994, 343, 1634-1635. 10. Cover T.L., Puryear W., Perez- Perez G.I., Blaser M.J.: Effect of urease on HeLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect. Immun. 1991, 59, 1264-1270. 11. Lehmann F.S., Stalder G. A.: Hypotheses on the role of cytokines in peptic ulcer disease. Eur. J. Clin. Invest. 1998, 28, 511-519. 12. Kalia N., Morton D., Jakobs S., Brown N.J., Reed M.W.R., Bardhan K.D.: The role of platelets and mast cells in Helicobacter pylori - induced changes in rat gastric mucosal microcirculation in vivo. Gastroenterology 1996, 110: 148a (Abstr.). 13. Sobhani I., Denizot Y., Vissuzaine C., Vatier J., Benveniste J., Lewin M.J.: Mignon M.: Significance and regulation of gastric secretion of platelet - activating factor (PAF- acether) in man. Dig. Dis. Sci. 1992, 37, 1583-1592. 14. Denizot Y., Sobhani I., Rambaud J.C., Lewin M., Thomas Y., Benveniste J.: PAF - acether synthesis by Helicobacter pylori Gut 1990, 31, 1242-1245.


