© Borgis - New Medicine 1/2001, s. 58-61
Elzbieta Maciorkowska1, Beata Szynaka2, Maciej Kaczmarski1, Andrzej Kemona2
Helicobacter pylori and chronic urticaria in children
1 IIIrd Department of Paediatrics, Medical Academy of Bialystok, Poland
Head: Professor Maciej Kaczmarski MD, PhD
2 Institute of Pathological Anatomy, Medical Academy of Bialystok, Poland
Head: Professor Boguslaw Musiatowicz MD, PhD
Summary
The authors carried out an ultrastructural evaluation of the gastric and duodenal mucosa in H. pylori-infected children with co-existing urticaria. In all cases, inflammatory cells such eosinophils and neutrophils, plasmocytes, lymphocytes and mast cells were found in the mucosal membrane under the epithelium, in the vicinity of vessels. The surface of mast cells subjected to degranulation had significantly fewer processes than the surface of undamaged cells. Granulocytes and plasmocytes were noticed in the vicinity of mast cells subjected to degranulation. There was a segmental loss in the endothelial lining and only the basement membrane determined the vessel continuity.
Introduction
The majority of studies indicate the significant role of H. pylori in the pathogenesis of chronic active gastritis and duodenitis (10, 11). The administration of H. pylori to volunteers causedhistologically-defined gastritis (4). Its eradication led to a regression of symptoms, while re-infection resulted in frequent recurrence of clinical ailments (5, 9). Although the precise mechanism through which H. pylori infection affects mucosa is not clear, this bacterium is considered to produce and release substances activating and attracting neutrophils which then cause tissue damage (4, 11, 23). The observation supporting this hypothesis proves that the severity of H. pylori infection depends on the size of a neutrophilic infiltration and the extent of mucosal damage (2).
Mai and Craig report that H. pylori extracts contain substances causing chemotactic activity in neutrophils and monocytes (7,16) and its water extracts enable leukocyte adhesion and their migration from the mesenteric veins (24). These phenomena suggest that H. pylori infection may lead to gastrointestinal mucosa damage through the initiation of an acute inflammatory reaction. Gastrointestinal inflammation is accompanied by changes in the structure and function of vessels, manifested in the increased microvascularpermeability of fluid and proteins (14).
Kurose et al. showed that microvascular malfunction in the inflamedmucosa is not only associated with leucocyte adhesion and migration butother myelocytessuch as mast cells and platelets, as well. They cause an increase in the permeability (14).
Kurose was the first to find out that H. pylori extracts may induce perivascular mast cell degranulation (14). This degranulation was already advanced10 minutes after the exposureof the mesenteric cells to H. pylori hydric extract.
The authors evaluated the significant influence of Ketotifen, which limitedthe degranulation of H. pylori hydric extract-induced mast cells and prevented early vascular albumin exudate.
Activated mast cells release pro-inflammatoryagents that can enhance microvascular permeability. They include platelet activating factor (PAF), leukotrienes (B4), histamine and tryptase. However, Bechi et al.prove that H. pylori extracts have no influence on the isolated mast cell, indicating that themechanism of mast cell degranulation in vivo is still unknown and needs further investigation (3).
Pink or porcelain urticarial wheals appearing rapidly and resolving within severalhours without any sign, result from the release of various mediators by degranulating mast cells in H. pylori - inflamed gastric mucosa. Urticarial changes are accompanied by itching.
An urticarial wheal is produceddue to the enhanced permeability of vessels and swelling caused by the release of histamine and other mediators including neuropeptides.
The purpose of our study was the ultrastructural evaluation of the gastric and duodenal mucosa in H. pylori - infected children with chronic urticaria.
Methods
The studies were performed in 7 children aged 11-18 years (mean age 14.4 years) with chronic urticaria and dyspeptic symptoms of the gastrointestinal tract. There were 4 girls and 3 boys, and 2 children constituted controls. Children were hospitalised in the IIIrd Department of Paediatrics or treated in the Outpatient Gastrology Department. Endoscopic examinations of the upper gastrointestinal tractwere performed in all patients with chronic symptoms. During endoscopy the urease test (CLO-test) was made and mucuswas taken from the duodenum to exclude Giardia lamblia infection.
To evaluate gastric mucosa morphologically in each patient, 2 sampleswere obtainedfrom the pyloric segment at a distance of 2-3 cm from the pylorus, and from the corpus about 8 cm from the antrum. Two samples of mucosa were taken from the extra bulbar part of the duodenum. The samples were placed in 10% neutralized formalin.
Additionally, biopsy specimens were taken from antrum, corpus and duodenum for ultrastructural examination. They were fixed in 3.6% glutaraldehyde at 4o C for 2 hrs and postfixed in 2% osmium tetroxide for 1hrs. After dehydration in alcohol, they were washed in propylene oxide and embedded in Epon 812. The ultrathin sections were cut on a Reichert ultramicrotome, doubly stained with uranyl acetate and lead citrate, and examined using an electron microscope EM 900 PC (OPTON West Germany).
All examinations were carried out with the consent of the children´s parents and approved by the Committee of Ethics and Supervision over Human and Animal Examinations, Medical Academy of Bialystok.
Results and discussion
Many reports describe relationships between H. pylori infection and other diseases which are not related to the gastrointestinal tract, particularly H. pylori infection and vascular diseases (1,18). Some authors suggest a correlation between H. pylori infection and the occurence of urticaria and acne erythematosa (18).
All substances produced in H. pylori infection may play a role in the pathogenesis of diseases not associated with the gastrointestinal tract (1,18).
Helicobacter pylori infection may have a direct or indirect effect by triggering immune andinflammatory responses (1). Cytokines and other cell mediators of an acute stage can be especially connected with vasomotor disorders.
In 1972, when H. pylori and its association with gastric mucosa inflammation were unknown,von Gloor showed gastric dysfunction in patients with chronic urticaria (25).
Juhlin et al. in 1981 described dyspeptic symptoms in 44% of patients with recurrent chronic urticaria (12).
In 1994 Kolibasova et al (13) associated the regression of dyspeptic symptoms with H. pylori eradication, and Tebbe (1996) notedcomplete regression of urticaria in 47% and partial in 35% of patients after eradication therapy (21).
Liutu was the next author to relate H. pylori infected gastric mucosa inflammation to urticaria (1998); 43 of a total of 53 patients with urticaria had H. pylori infection. After successful H. pylori eradication in 32 patients urticaria symptoms did not reappear in 78%during the 6 months´follow-up (15).
Our patients presented no symptoms of recurrent urticaria during 6months´ monitoring (15). After 6-8 weeks of treatment gastroscopy showed normal gastric and duodenal mucosa.
Morphological examinations of gastric, antral and corpus mucosa revealed moderate or severe H. pylori infection. Duodenal mucosa showed a chronic inflammatory state without intestinal villus atrophy.
Ultrastructural evaluation of gastric and duodenal mucosa in H. pylori children showed no damage. Mast cells present in the mucosa proper in the vicinity of blood vessels contained numerous granules of high electron density encircled by a membrane. The cell surface was well-developed and contained a number of thin processes.
In H. pylori-infected children numerous bacteria placed close or adherent to epithelial villi were found on the surface of the gastric mucosa, mainly inpits (more frequently in the antrum than in the corpus), and on the surface of duodenal villi (Photo 1).
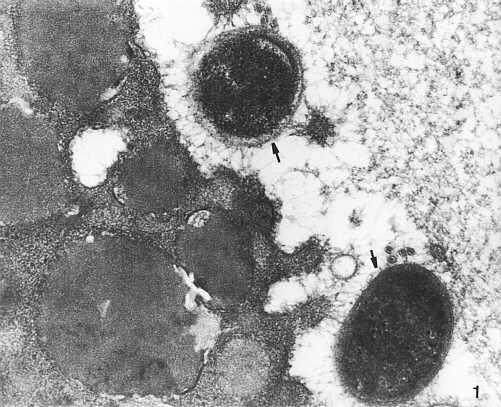
Photo 1. Helicobacter pylori bacteria (?) in the close vicinity of mucosal microvilli. TEM x 20,000.
In bacterial colonization sites, smoothing of the epithelial cell surface or cell membrane damage was observed. The cytoplasm of numerous cells showed vacuoles of differentsizes filled with medium - dense microgranular material. Some cells were damaged by H. pylori (Photo 2).
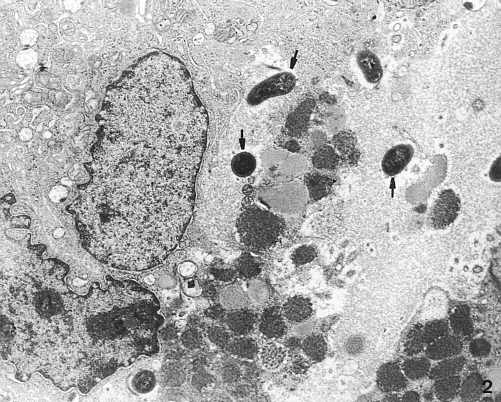
Photo 2. Helicobacter pylori bacteria (?) between damaged gastric mucosa cells. TEM x 4,400.
In all cases, inflammatory cells such as: eosinophils and neutrophils, plasmocytes, lymphocytes and mast cells were found in themucosal membrane under the epithelium in the vicinity of vessels. In one case, inflammatory infiltrates co-existed with fibroblasts.
Mucosal mast cells presented features of degranulation: ghost-like granules or filled with membrane residue encircled by light space. Some granules contained scrooll-like, coiled membranes, while others of medium electron density appeared homogenic or patchy (Photo 3).

Photo 3. Partly degranulated mast cell (M c) with numerous ghost-like granules (?) containing coiled membranes (T) which appear patchy (ä). Fragment of plasmatic cell visible in the vicinity of mast cell (P) TEM x 7,000.
The surface of mast cells subjected to degranulation had significantly fewer processes thanthe surface ofnon-degranulated cells (Photo 3).
Granulocytes and plasmocytes were observed in the close vicinity of mast cells subjected to degranulation (Photo 3).
Platelet aggregations with granulocytes and lymphocytes were observed in the vascular lumen. There was a segmental loss in the endothelial lining and only the basement membranedetermined the vessel continuity.
Human mast cells are placed along the gastrointestinal tract and localized mainly in lamina propriawhich is responsible for absorption and contributes to immunological processes. The epithelium coveringmucosa which remains in close contact with the intestinal contentsis also involved (20).
Submucosal mast cells were found in the vicinity of blood and lymphatic vessels (19). Their presence was confirmed in the third month of foetal life and their number increases with foetal development and decreases with puberty (9). Mast cells in mucosal lamina propria differ from those in connective tissue inlight staining with toluldine blue, IgE antibodies on the surface, smaller size of cytoplasm granuleswhich are more heterogenious and are of lower density.
The cells present in intestinal epithelium may constitute subpopulation III of mast cells, apart from the cells of connective tissue and lamina propria. It is not known if they are less mature or partly devoid of their granulation during their passage to intestinal epithelium (8).
The release of histamine from mast cells occurs with the involvement of antibodies. IgE binds with the membrane receptors of mast cells initiating biochemical changes which inducehistamine release.
According to Therp gastrin may induce the release of histamine from the mast cells localized in the skin (22). Other substances causing the release of histamine are neuropeptides, parts of complement, kinines and macrophage-derived agents.
Plasmatic cells producing IgE in the gastric and intestinal mucosa are more concentrated in theulceric regions (1). IgE mediated responses may be essential in the pathogenesis of mast cell markers´ release. Studies of animal models have proved that gastric IgE response to proteins included in food-induced mast cell degranulation causing histamine release and increased gastric acid secretion (17).
Aceti et al. proved the presence of specific IgE antibodies in the serum of infected patients and in vitro release of histamine from basophils of patients (1).
The release of H. pylori specific IgE - dependent histamine was not confirmed (17).
Mast cells are the source of many other pro-inflammatory mediators and cytokines. These include heparin, chondroitin sulphate, proteases, eosinophilic chemotactic factor. After degranulation stimulated by IgE-related antigen these mediators activated by complement particles or other substances, contribute to the recruitment and activation of other mucosa cell populations (17). Afterdegranulation mast cells secrete new mediators like prostaglandin D2, leukotriens, C4,D4, E4, platelet activating factor and IL-8. However IL-4, IL-5, IL-6 and TNF-a are secreted by mast cells frompreviously and newly formed sources (22).
Mast cell degranulation in the gastric mucosa of ulceric patients, observed using an electron microscope and H1 antagonisttherapy reducing symptoms in nonulceric dyspepsia together with the increasednumber of mast cells in gastric bioptates suggest that mast cells may play a certain role in gastric mucosa dysfunction. Additionally, they may cause distant extra-gastrointestinal tract symptoms, such as chronic urticaria not subjectedto a conventional treatment.
Conclusions
1. Helicobacter pylori infection present in children with chronic urticaria, ultrastructural changes and certain features of mast cell degranulation indicate a likely a etiopathogenic role of these cells.
2. The relationship between urticaria and H. pylori infection may be of clinical importance, indicating a likely a ethiologic factor in chronic urticaria.
Piśmiennictwo
1. Aceti A et al.: Basophil-bound and serum immunoglobulin E directed against Helicobacter pylori in patients with chronic gastritis. Gastroenterology, 1991, 101(1), 131-137. 2. Bayerdorffer E et al.: Difference in expression of Helicobacter pylori gastritis in antrum and body. Gastroentrology 1992, 102, 1575-1582. 3. Bechi P et al.: Helicobacter pylori potentiates histamine release from serosal rat mast cells in vitro. Dig Dis. Sci. 1993, 38, 944-949. 4. Blaser MJ: Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology 1992, 102, 720-727. 5. Cover TL, Blaser MJ: Helicobacter pylori and gastroduodenal disease Annu. Rev. Med. 1992, 43, 135-145. 6. Crabtree JE: Immune and inflammatory responses to Helicobacter pylori infection. Scand. J. Gastroenterol Suppl . 1996, 215. 7. Craig PM et al.: Helicobacter pylori secretes a chemotactic factor for monocytes and neutrophils. Gut 1992, 33, 1020-1023. 8. Dyduch A et al.: The role of histamine in gastrointestinal tract in physiology and pathology states. Pediatr. Pol. 1996, 5, 391-354. 9. Goodwin CS, Armstrong JA,Marshall BJ: Campylobacter pyloridis, gastritis, and peptic ulceration. J. Clin. Pathol. 1986, 39, 353-365. 10. Graham DY: Helicobacter pylori: its epidemiology and its role in duodenal ulcer disease. J. Gastroenterol. Hepatol. 1991, 6, 105-113. 11. Graham DY: Pathogenic mechanisms leading to Helicobacter pylori-induced inflammation. Eur. J. Gastroenterol. Hepatol 1992, 4, 9-16. 12. Julhin L: Recurrent urticaria: clinical investigation of330 patientss. Br J. Dermatol 1981, 104, 369-381. 13. Kolibasova K Von, Cervencova D, Hegyi E et al.: Helicobacter pylori-ein moglicher atiologischer factor der chronischen urticaria . Dermatosen 1994, 42, 235-236. 14. Kurose I et al.: Helicobacter pylori-induced microvascular protein leakage in rats: role of Neutrophils, mast cells and platelets Gastroenterology 1994, 107, 70-79. 15. Liutu M et al.: Chronic urticaria and Helicobacter pylori infection. J. Dermatol Treatment, 1998, 9, 31-33. 16. Mai UE et al.: Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J. Clin. Invest 1991, 87, 894-900. 17. Nakajima S et al.: Mast cell involvement in gastritis with or without Helicobacter pylori infection. Gastroenterology 1997, 113(3), 746-754. 18. Rebora A, Drago F, Parodi A: May Helicobacter pylori be important for dermatologists. Dermatology 1995, 191,6-8. 19. Saavedra-Delgado AM, Metcalf DD: The gastrointestinal mast cell in food allergy. Ann. Allergy. 1983, 51, 185-189. 20. Strobel S, Miller HR, Ferguson A: Human intestinal mucosal mast cells: evaluation of fixation and staining techniques. J. Clin. Patol. 1981, 34, 851-855. 21. Tebbe B et al.: Helicobacter pylori infection and chronic urticaria. J. Am. Acad Drematol 1996, 34, 685-686. 22. Tharp MD, Thirby R, Sullivan TJ: Gastrin induces histamine release from human cutaneous mast cells. J. Allergy Clin. Immunol. 1984, 74, 159-165. 23. Wallace JL: Possible mechanisms and mediators of gastritis associated with Helicobacter pylori infection. Scand J. Gastroenterol Suppl. 1991, 187, 65-70. 24. Yoshida N et al.: Mechanisms involved in Helicobacter pyloriinduced inflammation. Gastroenterology 1993, 105, 1431-1440. 25. Von Gloor M, Heinkel K, Schuluz U: Zur pathogenetischen Beteutung von magenfunktions-stOrungen bei allergisch betingter, chronischer urticaria. Dermatol Monatsschr 1972, 158, 96-102.


