*Melinda Pènzes1, Pèter Balázs1, Kristie L. Foley2
Changes in smoking-related health knowledge and smoking status of Hungarian adolescents
1Institute of Public Health, Faculty of Medicine, Semmelweis University, Budapest, Hungary
Head of Institute: prof. Károly Cseh, MD, PhD
2Department of Social Sciences and Health Policy, Wake Forest University, Winston-Salem, NC, USA
Head of Department: prof. Kristie L. Foley, PhD
Summary
Introduction. School-based anti-smoking programs often educate adolescents about the adverse health effects of smoking to enable them to make informed decisions about smoking.
Aim. Our study aimed to assess changes in smoking-related health knowledge and smoking status in two cohorts of adolescents.
Material and methods. A three-year prospective survey (from 2010 to 2012) with annual data collection was conducted in Hungary’s six metropolitan cities among randomly selected elementary (younger cohort) and secondary school (older cohort) students (N = 1,092; 54% females). Measures included a 14-item scale for smoking-related health knowledge and variables to assess demographics and self-reported smoking status.
Results. The prevalence of past month smoking increased by 8.8% in the younger cohort (p < 0.001) and 11.0% in the older cohort (p < 0.001). Throughout the study, 69.4% of the sample remained non-smoker, 12.8% remained smoker, 13.9% initiated smoking and 3.8% quit smoking. Smoking-related health knowledge changed significantly during three years with inconsistent positive and negative changes. Overall knowledge increased at a higher rate among students in the younger cohort. Those initiating smoking showed the lowest level of knowledge at the last wave of data collection.
Conclusions. To reduce smoking initiation and to promote quitting, comprehensive prevention strategies should be conducted, such as school-based programs that incorporate skill-building (not exclusively health education) and peer-focused anti-smoking programs.
INTRODUCTION
The prevalence of adolescent smoking shows unfavorable trends in Hungary. Two-thirds of youth have tried cigarette by the age of 16 and 35-39% of them were current smokers (past 30 days) (1, 2). Education about the adverse health effects of smoking aimed to raise awareness of negative health consequences and prevent initiation is a cornerstone of many school-based tobacco prevention programs (3, 4). Schools provide an optimal social environment to influence adolescent smoking behavior because most children can be reached there and prevention fits well into educational and holistic school health promotion requirements (5, 6). In the past decades, numerous school-based tobacco and other drug prevention programs were implemented in Hungarian schools starting predominantly in the 5th class of elementary schools. However, little is known about the frequency, content and effectiveness of these interventions (7).
Recently, a nationwide study found decreasing yearly participation rates of 13-15 years old adolescents in school-based anti-smoking programs (8). A general review of Hungarian school-based drug prevention programs indicated that the interventions are methodologically heterogeneous, poorly designed, and non-professionally implemented and focused primarily on increasing health literacy of legal and illicit drug use (4, 7). Hungarian adolescents also report that anti-smoking interventions disproportionally overemphasized the adverse health consequences of smoking (9). However, there is little evidence to support the beneficial effects of this strategy on adolescents’ smoking behavior (4, 5). Seemingly, information-giving prevention programs may increase the awareness of adverse health effects of smoking, but have limited or no impact on the youth’s long term, smoking-related decisions (3, 4).
AIM
Our 3-year prospective study aimed to 1) assess changes of smoking status among Hungarian adolescents; and 2) explore differences in their smoking-related health knowledge over time.
MATERIAL AND METHODS
Participants and procedure
A 3-year prospective cohort study with yearly data collection started in the second half of the 2009-2010 school year in Hungary’s six metropolitan cities (Budapest, Debrecen, Győr, Miskolc, Pècs, Szeged). We used a cluster random sampling strategy, stratifying by number of students and school types of the settlements using annual data (2008) of the Public Education Information Office. Among 413 invited schools (elementary, vocational and high schools), 78 agreed to participate in the prospective survey. We invited 2,985 students to participate in the baseline survey. Parents were informed by a passive consent procedure; 418 refused participation, thus 2,567 students were informed both verbally and in writing about the voluntary nature of their participation of which 86.0% participated. Trained data collectors unknown to the students requested them to complete the self-administered questionnaire within one teaching hour. Data were entered anonymously, using a separate name-identifier linkage document. At baseline, randomly selected 6th (younger cohort) and 9th graders (older cohort) completed the survey (N = 2,208). Due to the prospective nature of the study, loss to follow-up occurred and 49.5% of baseline participants (N = 1,092) were successfully tracked over three years. The study was approved by the Institutional Review Board of Semmelweis University, Budapest (No.: 104/2009).
Measures
Demographic variables: gender (female/male), age in years and school grade were reported at each data collection waves (baseline or Time 1 = T1; follow-up 1 or Time 2 = T2; follow-up 2 or Time 3 = T3). Elementary school participants were 6th, 7th and 8th graders, while secondary school students were 9th, 10th and 11th graders at T1, T2 and T3, respectively.
Cigarette smoking status: Self-reported past 30 days smoking frequency. Participants who smoked ≥ 1 cigarette in the past month were regarded as current smokers (10). Smoking status were assessed from T1 to T3 and four categories were considered for analyzes (non-smokers, current smokers, students who initiated, and quitters).
Smoking-related health knowledge: fourteen, medically established health consequences of smoking were itemized (fig. 1) and respondents were requested to indicate those they think to be associated with smoking (11). We created a “knowledge scale for health consequences of smoking” (knowledge scale) by summing responses (maximum score: 14). Cronbach’s α-s, a measure of internal consistency of a scale, showed acceptable or good reliability (T1: 0.76; T2: 0.81; T3: 0.83).
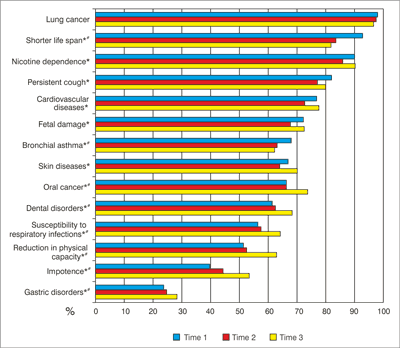
Fig. 1. Changes in the knowledge of smoking-related adverse health consequences (N = 1,075).
*p < 0.001 (Cochran’s Q test)
#p ≤ 0.010 (McNemar’s test to test the difference between T1 and T3)
Analyses
Descriptive statistics (Pearson’s chi-square test, mean and SD, Mann-Whitney U-test) were used to describe the sample. McNemar’s test and Cochran’s Q test with Bonferroni correction of p-value (for three comparisons, significant at p < 0.017) was applied to compare categorical variables of pairwise or overall survey waves. Two-way mixed ANOVA analyses with Tukey’s test were performed to identify changes in the mean value of knowledge scale at each survey time period. Data were analyzed using SPSS 22.0 and ROPstat 2.0 statistical program packages (12).
RESULTS
Table 1 shows descriptive characteristics of the sample. More students from the older cohort (57.2%) were retained during the three-year prospective survey compared to the younger one (42.8%) and females were overrepresented among secondary school participants. At T3 compared to baseline, the prevalence of past month smoking increased by 8.8% among elementary school students (Q(2) = 38.6, p < 0.001) and 11.0% among secondary school respondents (Q(2) = 44.11, p < 0.001). Overall, the prevalence of current smoking was greater among girls compared to boys, although the difference was significant only at T3 (29.9 vs 23.0%, respectively; χ2(1) = 6.59, p = 0.010). Regarding smoking status, 69.4% of the sample maintained non-smoking behavior and 12.8% of participants were smokers throughout the prospective study; 13.9% initiated smoking and 3.8% quit smoking. Changes in smoking status differed significantly in the two age cohorts (χ2(3) = 108.18, p < 0.001) whereas the proportion of continuous smokers, quitters and students who initiated smoking was higher in the older cohort (20.6%, 5.1% and 16.2%, respectively) compared to the younger one (2.4%, 2.1% and 10.9%, respectively).
Table 1. Descriptive characteristics of the sample at baseline, follow-up 1 and follow-up 2 (N = 1,092).
| Variables | Baseline (T1) | Follow-up 1 (T2) | Follow-up 2 (T3) |
6th graders – younger cohort
(N = 467) | 9th graders – older cohort
(N = 625) | p-value1 | 7th graders – younger cohort
(N = 467) | 10th graders – older cohort
(N = 625) | p-value1 | 8th graders – younger cohort
(N = 467) | 11th graders – older cohort
(N = 625) | p-value1 |
| Gender, N (%) | | | | | | | | | |
| Male | 240 (51.4) | 260 (41.6) | 0.001 | 240 (51.4) | 260 (41.6) | 0.001 | 240 (51.4) | 260 (41.6) | 0.001 |
| Female | 227 (48.6) | 365 (58.4) | | 227 (48.6) | 365 (58.4) | | 227 (48.6) | 365 (58.4) | |
| Mean age in years (SD) | 12.00 (0.57) | 15.01 (0.59) | NA | 12.99 (0.61) | 16.03 (0.58) | NA | 14.08 (0.57) | 17.09 (0.57) | NA |
| Current smoker, N (%) | 21 (4.5) | 161 (25.8) | < 0.001 | 32 (6.9) | 205 (32.8) | < 0.001 | 62 (13.3) | 230 (36.8) | < 0.001 |
| Knowledge scale for health consequences of smoking, mean (SD) | 8.76 (2.89) | 9.98 (2.83) | < 0.001 | 8.46 (3.25) | 9.73 (3.24) | < 0.001 | 9.39 (3.27) | 10.13 (3.40) | < 0.001 |
NA – not applicable, 1p – value based on Pearson’s χ2-test and Mann-Whitney U-test
There were significant changes in knowledge of almost all smoking-related health consequences during the prospective survey (fig. 1). By T3, the change was significant and positive for adverse health consequences which were less known at baseline (oral cancer, dental disorders, susceptibility to respiratory infections, reduction in physical capacity, impotence, gastric disorders), while the knowledge of nicotine dependence, cardiovascular diseases and fetal damage increased non-significantly. Participants reported decreased knowledge for some of the adverse health consequences by T3 (shorter life span, bronchial asthma, lung cancer, persistent cough).
Two-way mixed ANOVA analysis indicated that both the main effect of time (F(2) = 20.29, p < 0.001) and cohort (F(1) = 47.39, p < 0.001) were significant (fig. 2). Tukey’s test demonstrated that the amount of knowledge decreased for T2, while increased for T3. The interaction between time and cohort was also significant (F(2) = 4.76, p = 0.009). The main effect of smoking status were not significantly associated with change in knowledge (F(3) = 0.17, p = 0.919), however, the main effect of time was significant (F(2) = 5.09, p = 0.006), and the interaction between smoking status and time was tendentious (F(6) = 2.04, p = 0.057) (fig. 3). The pattern of smoking-related knowledge change was similar in all four smoking groups, but students initiating smoking by T3 showed a decline in knowledge from T1 to T2 and their knowledge remained lowest for T3.
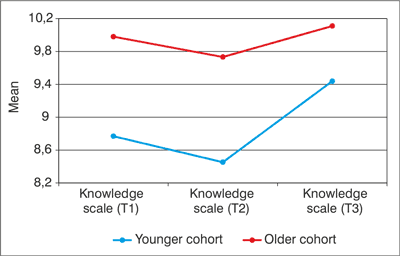
Fig. 2. Changes in the mean values of smoking-related health knowledge by age cohorts (N = 1,041).
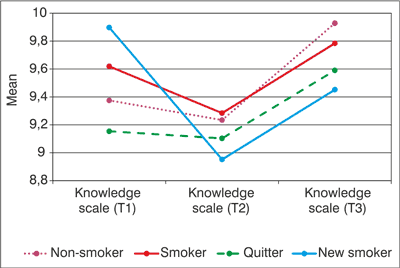
Fig. 3. Changes in the mean vaules of smoking-related health knowledge by smoking status (N = 1,041).
DISCUSSION
In our study, the overall knowledge of adverse health consequences of smoking changed positively and significantly among Hungarian adolescents over the 3-year study period, however, the directions of changes of specific smoking-related health consequences were inconsistent. Overall knowledge was significantly higher among older adolescents, although greater increases in knowledge occurred among the younger cohort. Significant differences were not detected by smoking status, similar to another study of Hungarian adolescents’ smoking behavior (13). Our results indicate that young adolescents who smoke have broad health knowledge of smoking-related consequences, consistent with prior reports (3). Mazanov et al. found that adolescent smokers were more health literate than non-smokers (3). Despite proper awareness of adverse effects of smoking in early adolescence in our sample, such knowledge did not deter children from smoking initiation. Smoking prevention programs often do not reach high risk subgroups of youth or do not emphasize the consequences of smoking as one of the greatest concerns for the underage population (14). Current information-only school-based anti-smoking programs, therefore, should not be expected to demonstrate a relationship between tobacco-related health knowledge and smoking initiation or cessation among youth (3, 5).
We are aware of limitations of this study. First, attrition presented a major concern with almost half of our sample being lost to follow-up, with a significantly greater proportion of smokers and secondary school students being more likely to be lost (9). Nonetheless, the sample of over 1,000 adolescents in different age cohorts allows us to meaningfully assess changes of smoking behavior and tobacco-related health knowledge among Hungarian students. Second, self-reported data by recall bias should be treated with caution. However, high degree of concordance has been found between self-reported and biochemically validated smoking status of adolescents (15). Third, our findings may have limited generalizability to rural and non-Hungarian communities because our sample only included urban adolescents.
CONCLUSIONS
Increasing awareness of smoking-related adverse health consequences is necessary, but insufficient to enable adolescents to make informed decisions about their behavior related to tobacco use. There is little evidence that even professionally implemented, school-based intervention programs that focus solely on health consequences are effective at preventing smoking initiation or promote quitting (3-5). These programs should target high risk subgroups of adolescents (such as communities with lower socioeconomic status or other marginalized populations) with appropriate anti-smoking messages and should be combined with programs using social competence and social influence approaches (4, 5, 14).
ACKNOWLEDGEMENT
The authors thank to Edit Czeglèdi PhD her assistance in statistical analyses.
This publication was made possible by Grant Number 1 R01 TW007927-01 from the Fogarty International Center, the National Cancer Institute, and the National Institutes on Drug Abuse, within the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Piśmiennictwo
1. Halmai R, Nèmeth Á: Dohányzási szokások. [In:] Nèmeth Á, Költő A (eds.): Serdülőkorú fiatalok egèszsège ès èletmódja 2010. Országos Gyermekegèszsègügyi Intèzet, Budapest 2011. 2. Hibell B, Guttormsson U, Ahlström S et al.: The 2011 ESPAD Report. Substance use among students in 36 European countries. The Swedish Council for Information on Alcohol and Other Drugs, Stockholm 2012. 3. Mazanov J, Byrne D: Changes in adolescent smoking behaviour and knowledge of health consequences of smoking. Aust J Psychology 2007; 59: 176-180. 4. Nádas E, Rácz J, Urbán R: A szerfogyasztás megelőzèsère irányuló iskolai egèszsègfejlesztèsi programok szakmai útmutatója. Egèszsègügyi Minősègfejlesztèsi ès Kórháztechnológiai Intèzet, Budapest 2009. 5. Thomas RE, Perera R: School-based programmes for preventing smoking. Cochrane Database Syst Rev 2006; 3: CD001293. 6. 20/2012. (VIII. 31.) EMMI rendelet a nevelèsi-oktatási intèzmènyek működèsèről ès a köznevelèsi intèzmènyek nèvhasználatáról; http://net.jogtar.hu/jr/gen/hjegy_doc.cgi?docid=A1200020.EMM (10.09.2015). 7. Paksi B, Demetrovics Zs, Nyírády A et al.: A magyarországi iskolai drogprevenciós programok jellemzői. Addiktológia 2006; 5: 5-36. 8. Balku E, Demjèn T, Kimmel Zs, Vitrai J: Nemzetközi ifjúsági dohányzás felmèrès, Egèszsègügyi Világszervezet: összefoglaló tanulmány, 2013. Dohányzás Fókuszpont, Országos Egèszsègfejlesztèsi Intèzet 2013. 9. Pènzes M: Adolescent tobacco smoking: facts, knowledge and opinions based on a prospective study. [In:] Balázs P (ed.): Increasing capacity for tobacco research in Hungary 2008-2013. Magyar Tudománytörtèneti Intèzet, Budapest 2013. 10. Centers for Disease Control and Prevention: 2009 Youth Risk Behavior Survey (YRBS) data users manual; http://www.cdc.gov/healthyyouth/data/yrbs/data.htm (03.06.2010). 11. Mackay J, Eriksen M: The tobacco atlas, first edition. World Health Organization, Geneva, Switzerland 2002. 12. Vargha A, Torma B, Bergman LR: ROPstat: a general statistical package useful for conducting person-oriented analyses. J for Person-Oriented Research 2015; 1: 87-98. 13. Urbán R, Demetrovics Zs: Smoking outcome expectancies: a multiple indicator and multiple cause (MIMIC) model. Addict Behav 2010; 35: 632-635. 14. Marti J: A best-worst scaling survey of adolescents’ level of concern for health and non-health consequences of smoking. Soc Sci Med 2012; 75: 87-97. 15. Post A, Gilljam H, Rosendahl I et al.: Validity of self reports in a cohort of Swedish adolescent smokers and smokeless tobacco (snus) users. Tob Control 2005; 14: 114-117.


