Karolina Raczkowska-Łabuda, Anna Gorzelnik, Jolanta Jadczyszyn, *Lidia Zawadzka-Głos
HPV-related head and neck tumors – 10 years of experience with human papillomavirus vaccinations
HPV-zależne nowotwory głowy i szyi a 10 lat doświadczeń ze szczepionkami przeciwko wirusowi brodawczaka ludzkiego
Department of Pediatric Otolaryngology, Medical University of Warsaw, Poland
Head of Department: Associate Professor Lidia Zawadzka-Głos, MD, PhD
Streszczenie
W ostatnich latach obserwuje się w Europie dynamiczny wzrost zachorowań na nowotwory głowy i szyi. Co roku około 6000 osób w naszym kraju zostaje postawionych przed diagnozą patologicznego rozrostu w obrębie narządów głowy i szyi. Ryzyko zachorowania na nowotwory tej grupy zwiększa się u obu płci proporcjonalnie do wieku, ze szczytem około 64. r.ż. Prawidłowo postawiona diagnoza nowotworu głowy i szyi typowo ma miejsce w wieku między 45. a 64. r.ż.
W wielu krajach Europy, w tym i w Polsce, obserwowany jest tzw. „fenomen epidemiologiczny” czyli wzrost zapadalności na nowotwory głowy i szyi u osób poniżej 40. r.ż., które nigdy nie paliły i nie nadużywały alkoholu. Zmienił się profil pacjenta z nowotworem głowy i szyi.
Szacuje się, że ponad 90% mężczyzn i 80% kobiet będzie zainfekowanych przynajmniej jednym typem wirusa HPV w trakcie swojego życia. Połowę tych infekcji wywołają typy onkogenne. Typ 16 wirusa odpowiada za 90% infekcji wysokiego ryzyka i podnosi prawdopodobieństwo wystąpienia nowotworu jamy ustnej i gardła zależnych od wirusa brodawczaka ludzkiego nawet 15 do 230 razy. W obliczu odmienności przebiegu i rokowania u chorych ze zmianą związaną z wirusem brodawczaka ludzkiego wyłączono nowotwory głowy i szyi HPV-zależne z dotychczasowego systemu stagingu TNM (ang. tumor, nodes, metastasis) i utworzono odrębną grupę uwzględniającą jedynie zmiany tego typu.
Szczepionki przeciwko HPV to jeden z najważniejszych kroków w prewencji nowotworowej ostatnich kilku dekad. W Polsce dostępne są 3 różne preparaty ujęte jako dodatkowe w PSO.
Summary
In the recent years, there has been a rapid increase in the incidence of head and neck cancers in Europe. Every year, 6,000 persons in Poland are diagnosed with a neoplastic proliferative disease in the head and neck region. The risk of cancer of this group increases with age in both genders, with a peak incidence around the age of 64. A diagnosis of a head and neck tumor typically occurs between the age of 45 and 64.
In many European countries, including Poland, the so-called “epidemiological phenomenon” is observed. Epidemiological phenomenon consists in an increasing incidence of head and neck cancers in persons under 40 years of age who had never smoked or abused alcohol. The profile of patient with head and neck cancer has changed.
It is estimated that over 90% of men and 80% of women will be infected with at least one type of HPV in their lifetime. Half of the infections are caused by the oncogenic types. HPV type 16 is responsible for 90% of high-risk infections and increases the likelihood of human papillomavirus-related oropharyngeal cancers by 15 to 230 times, depending on the type of the virus. In the view of the differences in course and prognosis in patients with HPV-related cancers, these tumors have been excluded from the existing TNM staging system (tumor, nodes, metastasis), and a separate classification, only for this group of tumors, was created.
HPV vaccines have been one of the most important steps in cancer prevention in the last few decades. In Poland, 3 different vaccines are included in the National Vaccination Program as recommended, but they are not reimbursed by the State.
Introduction
In the recent years, there has been a rapid increase in the incidence of head and neck cancers in Europe, with Poland being no exception. Every year, approximately 6,000 persons in Poland are diagnosed with a neoplastic proliferative disease in the head and neck region, which are generally associated with unfavorable prognosis (1). The risk of cancer of this group increases with age in both genders, with a peak incidence around the age of 64. A diagnosis of a head and neck tumor typically occurs between the age of 45 and 64 (1, 2).
In many European countries, including Poland, the so-called “epidemiological phenomenon” is observed. Epidemiological phenomenon consists in an increasing incidence of head and neck cancers in persons under 40 years of age who had never smoked or abused alcohol (2, 3). In these cases, undisputable association of the disease with the infection with HPV (human papillomavirus) was detected (2, 3).
Epidemiology
According to the current data by the National Tumor Registry (pol. Krajowy Rejestr Nowotworów), head and neck tumors (i.e. diseases with IDC-10 codes C00-C15, C30-C33, C69, and C73) accounted for 9% of all malignancies diagnosed in males and 5% of all malignancies diagnosed in females in 2012 (1). Specialists treating these cancers suggest that these statistics are underestimated (4). WHO data show that 550,000 new cases of oropharyngeal cancer and 160,000 new cases of laryngeal cancers are diagnosed worldwide each year (2-6). It is estimated that approximately 400,000 persons worldwide die each year from head and neck tumors (2-6).
HPV epidemic?
In most countries of the world, the number of new cases of oral, laryngeal and hypopharyngeal cancer is decreasing, which is strongly associated with a decline in consumption of tobacco products. Unfortunately, in the past 20-30 years, the incidence of oropharyngeal malignancies (primarily of the tonsils, tongue base and velum) has been increasing. This seems to be confirmed by statistical data from Australia (7), Canada (8), Denmark (9), Norway (10), Sweden (11), Netherlands (12), United States of America (13), and United Kingdom (14). The analysis of the dissociation of the incidence of head and neck cancer in those two locations contributed to establishing a relationship between pharyngeal lesions and human papillomavirus (15). It is currently estimated that nearly 70% of cases of pharyngeal cancer is related to HPV (16, 17).
Profile of patients with an HPV-related head and neck cancer
As a result, the profile of a patient with an HPV-related head and neck tumor has changed as well. The majority of clinical data on the subject were collected in the ICON-S analysis (Collaboration on Oropharyngeal Cancer Network for Staging) (18). The study included 1,907 persons with HPV-related oral and pharyngeal tumors and 696 patients with squamous cell head and neck carcinomas that were not HPV-related. It was shown that a typical patient diagnosed with an HPV-related tumor is a young Caucasian male (with two peaks of incidence – at 30 and 55 years of age). Among the patients with HPV-related head and neck cancer, 84% of them are male, whereas in case of non-HPV related head and neck cancers, 76% of the patients are male. A typical patient with HPV-related head and neck cancers is not addicted to tobacco or alcohol (he consumes alcohol only occasionally), while a significant proportion of patients take marijuana. This observation does not change the fact that smoking increases the risk of infection with human papillomavirus (19). Patients with HPV-related tumors generally have a higher overall number of sexual partners (> 9) and oral sex partners (> 4) than HPV-negative patients (20). The most accurate data on race, ethnicity and sex of patients with HPV-related head and neck cancers concern United States of America (fig. 1) (21).
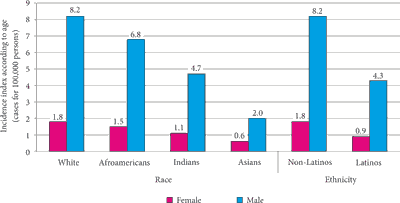
Fig. 1. Ethnical characteristics of patients with HPV-dependent head and neck cancer (21)
Types of human papillomavirus in laryngological cancers
It is estimated that more than 90% of men and 80% of women will be infected with at least one type of HPV during their lifetime (22-24). Half of these infections are caused by the oncogenic types of the virus (22-24). HPV type 16 is responsible for 90% of high-risk infections and increases the likelihood of human papillomavirus-related oral and pharyngeal cancers by 15 to 230 times, depending on the type of the virus (18, 25). Currently, no effective treatment for active HPV infection is known, and the course of infection is, in most cases, asymptomatic and self-limiting – recovery usually takes place within 1 to 2 years. Persons who are unknowingly infected transmit the pathogen to their partners. In addition, head and neck lesions, due to their location, develop asymptomatically or result in non-specific symptoms, typical for upper respiratory tract infections (sore throat, pain on swallowing, hoarseness) (2, 4). Moreover, there is no effective screening method for HPV-dependent head and neck cancers. Swabs, perfect for organs such as cervix or anus, are very difficult to obtain in the diagnosis of head and neck lesions. It would be desirable to obtain the material from deep crypts of palatine tonsils, but this procedure has a high risk of unsuitable sample acquisition and of false negative results (18).
HPV vaccination against cancer
The above reported data highlight the importance of HPV vaccination in the prevention of head and neck cancer. The preliminary report of the NHANES study (National Health and Nutrition Examination Survey) confirms this statement (26). There were 2,627 persons aged 18 to 33 included in the study. After on average 4 years elapsed from HPV vaccination (with a 4-valent vaccine, active against types 6, 11, 16, and 18), oral lavage was acquired from the participants and analyzed for human papillomavirus. Active infection with HPV type 6, 11, 16, or 18 was detected in 0.11% of the subjects who received at least one dose of the vaccine, and in 1.61% of subjects who were not vaccinated (p = 0.008). The difference between the groups corresponds to the reduction in the incidence of these types of the virus by 88.2%. At the same time, the incidence of infection with 33 other HPV types, which were not covered by the vaccine, was similar in both groups: 3.98% vs. 4.74% (p = 0.24) (26).
Quoting Associate Professor Maura L. Gillison: “The HPV vaccine is one of the most important advances in cancer prevention in the last several decades. Parents who choose to have their children vaccinated against HPV should realize that the vaccine may provide additional benefits, such as prevention of oral HPV infections linked to oral cancers” (27).
Diagnostics
Outcomes of treatment of patients with head and neck tumors in Poland are highly unsatisfactory. This is related, among others, to the advanced stage of the disease at the time of diagnosis, which, in turn, is a consequence of the lack of adequate screening methods and diagnostic algorithms (2, 3). Nearly 90% of patients present a painless lesion in the neck at the time of diagnosis (28). In some patients, it is difficult to determine the tumor’s origin due to its small size, and affected lymph nodes are often cystic in nature, which results in non-diagnostic aspirates in biopsy. There are several reasons for delayed diagnosis:
– lack of oncological alertness due to negative history of tobacco smoking;
– lack of signs and symptoms in the early stages of the disease;
– inadequate oral and pharyngeal examinations due to lack of knowledge and lack of equipment;
– small tumor size – below detection point in imaging studies;
– low sensitivity of results of lymph node aspiration;
– random choice of location for intraoperative biopsy (29).
As of today, the golden standard in diagnostic of HPV infection is in situ hybridization or PCR detecting viral DNA. However, in clinical practice, immunohistochemical examination of protein p16 expression or real-time PCR to assess HPV-16 viremia are widely used.
Protein p16 is a tumor-suppressing factor acting by binding cyclin D1 (CDK4/CDK6 complex), which prevents the phosphorylation of the Rb protein. In case of HPV-related cancers, an overexpression of the protein is observed. This indicator is highly sensitive – false negative results amount for less than 4% of the cases (30). High nuclear and cytoplasmic p16 expression strongly suggests the presence of HPV-related tumor. In case of moderate positive or weak positive results, ISH (in situ hybridization) or RT-PCR (real-time PCR) is advised (30). What is more, high titer of p16 is associated with better prognosis in HPV-related cancer (31). However, the possibility of receiving false positive results in testing for p16 expression should be considered, as it is not a specific marker for human papillomavirus marker, and is also present in 5-8% of HPV-unrelated head and neck cancers (32).
Prognosis
There are many reports in the literature confirming that patients with HPV-related head and neck cancer have better prognosis that patients with HPV-unrelated head and neck cancer (33-36). This observation remains true even in case of recurrence or advanced stage of cancer. Retrospective analysis on a large group of patients reported that overall 3-year survival rate in patients with head and neck tumor was 82.4% for patients with HPV-related lesion vs. 57.1% in patients with other head and neck cancer. The 3-year progression-free rate was 73.7% vs. 42.4%, respectively. This translates into 58% reduction in the risk of death and 51% reduction in the risk of progression (37).
cTNM and pTNM scale
In the view of the differences in course and prognosis in patients with HPV-related cancers, they have been excluded from the existing TNM staging system (tumor, nodes, metastasis), and a separate classification, only for this group of tumors, was created (8-18).
The 8th edition of TNM staging system developed by UICC (Union for International Cancer Control) and AJCC (American Joint Committee on Cancer) in 2017 distinguished HPV-related oropharyngeal cancer (tab. 1, 2) (38, 39).
Tab. 1. Clinical TNM for HPV-dependent head and neck cancer
| HPV related oropharyngeal carcinoma TNM clinical staging AJCC UICC 2017 |
| Primary tumor (T) |
| T category | T criteria |
| T0 | No primary tumor identified |
| T1 | Tumor 2 cm or smaller in greatest dimension |
| T2 | Tumor larger than 2 cm but not larger than 4 cm in greatest dimension |
| T3 | Tumor larger than 4 cm in greatest dimension or extension to lingual surface of epiglottis |
| T4 | Moderately advanced local disease. Tumor invades the larynx, extrinsic muscle of tongue, medial pterygoid, hard palate, or mandible or beyond. |
| Regional lymph nodes (N) – Clinical N (cN) |
| N category | N criteria |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | One or more ipsilateral lymph nodes, none larger than 6 cm |
| N2 | Contralateral or bilateral lymph nodes, none larger than 6 cm |
| N3 | Lymph node(s) larger than 6 cm |
| Distant metastasis (M) |
| M category | M criteria |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Prognostic stage groups – Clinical |
| When T is... | and N is... | and M is... | then the stage group is... |
| T0, T1 or T2 | N0 or N1 | M0 | I |
| T0, T1 or T2 | N2 | M0 | II |
| T3 | N0, N1, or N2 | M0 | II |
| T0, T1, T2, T3 or T4 | N3 | M0 | III |
| T4 | N0, N1, N2, or N3 | M0 | III |
| Any T | Any N | M1 | IV |
Tab. 2. Histopathological TNM histopatologiczny for HPV-dependent head and neck cancer
| HPV related oropharyngeal carcinoma TNM pathological staging AJCC UICC 2017 |
| Primary tumor (T) |
| T category | T criteria |
| T0 | No primary tumor identified |
| T1 | Tumor 2 cm or smaller in greatest dimension |
| T2 | Tumor larger than 2 cm but not larger than 4 cm in greatest dimension |
| T3 | Tumor larger than 4 cm in greatest dimension or extension to lingual surface of epiglottis |
| T4 | Moderately advanced local disease. Tumor invades the larynx, extrinsic muscle of tongue, medial pterygoid, hard palate, or mandible or beyond. |
| Regional lymph nodes (N) - Pathological N (pN) |
| N category | N criteria |
| NX | Regional lymph nodes cannot be assessed |
| pN0 | No regional lymph node metastasis |
| pN1 | Metastasis in four or fewer lymph nodes |
| pN2 | Metastasis in more than four lymph nodes |
| Distant metastasis (M) |
| M category | M criteria |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Prognostic stage groups - Clinical |
| When T is... | and N is... | and M is... | then the stage group is... |
| T0, T1, or T2 | N0 or N1 | M0 | I |
| T0, T1, or T2 | N2 | M0 | II |
| T3 or T4 | N0, N1 | M0 | II |
| T3 or T4 | N2 | M0 | III |
| Any T | Any N | M1 | IV |
Treatment
The distinction of this group of tumors from the previous classification is a huge advance, but does not result in changes in treatment regimens. The algorithm of treatment in patients with head and neck cancer is identical for patients with HPV-dependent and independent lesions. The current guidelines recommend multimodal approach, including chemotherapy (CT), radiotherapy (RT), and surgery. The long-term effects of such treatment include complications, such as mucositis, dysphagia, radiation-induced fibrosis, xerostomia, or dental problems. Research on deintensification of CT and RT is conducted. The ECOG 1308 (Eastern Cooperative Oncology Group) multi-center study conducted on 80 patients compared the efficacy of a lower dose RT (54 Gy in 27 fractions) in patients in stage III or IVA of HPV-related oropharyngeal cancer. Initially, all the patients received induction CT consisting of three cycles of cisplatin, paclitaxel, and cetuximab. Lower doses of RT were offered only to patients with a full clinical response to induction chemotherapy – 56 patients. Out of the 56 patients, 51 continued chemotherapy with cetuximab and 54 Gy radiotherapy. Other patients received 69.3 Gy in 33 fractions. In both groups, a weekly dose of cetuximab was included. In the group of patients with full response to induction CT, 2-year progression-free rate was 80%. In the same groups, 2-year survival rate was 94% (40). Nevertheless, as Beitler noted during the expert debate in Sea Island in 2015, E1308 did not reach its target goal of illness-free period of more than 2 years (40).
The greatest hope for optimizing therapy for patients with HPV-related head and neck cancer is placed in two analyzes: ECOR 331 and NRG HN002 (41, 42). The current trend for reducing the intensity of treatment is a step into the unknown and the fear of worsening the excellent survival rates that are possible under current therapeutic standards. Further multi-center studies are needed to reach a consensus on new treatment guidelines.
Conclusions
In the light of the above, the role of population HPV vaccination programs in prevention of head and neck cancer seems to be substantial. There is currently no effective screening method, moreover, there is no consensus on treatment methods, but the available HPV vaccination is very effective. Hence the necessity of pre-exposure immunization for both girls and boys.
Countries where population-based HPV vaccination programs were introduced are focusing on achieving the highest possible percentage of vaccinated population, popularizing knowledge human papillomavirus infection, and on publicizing the undeniable benefits of immunization of adolescents.
In Poland, we lost the battle to include HPV vaccination in the National Vaccination Program for the year 2017. No nationwide registry of HPV-dependent head and neck cancer is available, and the treatment outcomes are unsatisfactory.
Piśmiennictwo
1. Krajowy Rejestr Nowotworów. Głowa i szyja; http://onkologia.org.pl/nowotwory-narzadow-glowy-i-szyi/ (accessed 21.08.2017).
2. Program profilaktyki nowotworów głowy i szyi. Epidemiologia; http://www.pngs.wco.pl/epidemiologia/ (accesed 21.08.2017).
3. Od 5 lat edukują o nowotworach głowy i szyi. Gazeta Lekarska; http://www. gazetalekarska.pl/?p=36092.
4. Golusiński W: I Ogólnopolski Program Profilaktyki Nowotworów Głowy i Szyi. Świat Lekarza; http://swiatlekarza.pl/ogolnopolski-program-profilaktyki-nowotworow-glowy-szyi/.
5. Ferlay J, Soerjomataram I, Dikshit R et al.: Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359-E386.
6. Jemal A, Bray F, Center MM et al.: Global cancer statistics. CA Cancer J Clin 2011; 61(2): 69-90.
7. Hong AM, Grulich AE, Jones D et al.: Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets. Vaccine 2010; 28: 3269- 3272.
8. Auluck A, Hislop G, Bajdik C et al.: Trends in oropharyngeal and oral cavity cancer incidence of human papillomavirus (HPV)-related and HPV-unrelated sites in a multicultural population: The British Columbia experience. Cancer 2010: 116: 2635-2644.
9. Blomberg M, Nielsen A, Munk C et al.: Trends in head and neck cancer incidence in Denmark, 1978-2007: Focus on human papillomavirus associated sites. Int J Cancer 2011; 129: 733-741.
10. Mork J, Møller B, Dahl T et al.: Time trends in pharyngeal cancer incidence in Norway 1981-2005: A subsite analysis based on a reabstraction and recoding of registered cases. Cancer Causes Control 2010; 21: 1397-1405.
11. Hammarstedt L, Lindquist D, Dahlstrand H et al.: Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer 2006; 119: 2620-2623.
12. Braakhuis BJ, Visser O, Leemans CR: Oral and oropharyngeal cancer in the Netherlands between 1989 and 2006: Increasing incidence, but not in young adults. Oral Oncol 2009; 45: e85-e89.
13. Chaturvedi AK, Engels EA, Anderson W et al.: Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 2008; 26: 612-619.
14. Reddy VM, Cundall-Curry D, Bridger MW: Trends in the incidence rates of tonsil and base of tongue cancer in England, 1985-2006. Ann R Coll Surg Engl 2010; 92: 655-659.
15. Chaturvedi AK, Engels EA, Pfeiffer RM et al.: Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29: 4294-4301.
16. Mehanna H, Beech T, Nicholson T et al.: Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer – systematic review and meta-analysis of trends by time and region. Head Neck 2013; 35: 747-755.
17. Gillison ML, D’Souza G, Westra W et al.: Distinct risk factors profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 2008; 100: 407-420.
18. O’Sullivan B, Huang SH, Su J et al.: Development and validation of staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicenter cohort study. Lancet Oncol 2016; 17: 440.
19. Gillison ML, Broutian T, Pickard RK et al.: Prevelance of oral HPV infection in the United States 2009-2010. JAMA 2012; 307: 693-703.
20. Dahlstrom KR, Li G, Tortolero-Luna G et al.: Differences in history of sexual behavior between patients with oropharyngeal squamous cell carcinoma and patients with squamosus cell carcinoma at other head and neck sites. Head Neck 2011; 33(6): 847-855.
21. Viens LJ, Henley SJ, Watson M et al.: Human papillomavirus-associated cancers – United States, 2008-2012. MMWR 2016; 65(26): 661-666.
22. Chesson HW, Dunne EF, Hariri S, Markowitz LE: The estimated lifetime probability of acquiring human papillomavirus in the United States. STD 2014; 41(11): 660-664.
23. Hariri S, Unger ER, Sternberg M et al.: Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003-2006. J Infect Dis 2011; 204(4): 566-573.
24. Raczkowska-Łabuda K, Gorzelnik A, Zawadzka-Głos L: Why is a laryngologist interested in cervical cancer? Summary information about HPV vaccination. New Med 2017; 21(3): 84-93.
25. D’Souza G, Gross ND, Pai SI et al.: Oral human papillomavirus (HPV) infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol 2014; 32: 2408-2415.
26. Gillison ML, Broutian T, Graubard B et al.: Impact of HPV vaccination on oral HPV infections among young adults in the US. American Society of Clinical Oncology annual meeting. 2017.
27. Harris J: Vaccination Reduces Oral HPV Infections by 90%. Oncology Nursing News; http://www.oncnursingnews.com/web-exclusives/vaccination-reduces-oral-hpv-infections-by-90 (accessed 21.08.2017).
28. Garde SA, Kies MS, Morrison WH et al.: Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiat Oncol 2013; 8: 21.
29. Lewis A, Kang R, Levine A, Maghami E: The New Face of Head and Neck Cancer: The HPV Epidemic. Cancer Network; https: //www.cancernetwork.com (accessed 01.08.2017).
30. Lewis JS: P16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol 2012; 6(suppl. 1): S75-S82.
31. Weinberger PM, Yu Z, Haffty BG et al.: Molecular classification identifies a subset of human papillomavirus- associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 2006; 24: 736.
32. Liang C, Marsit CJ, McClean MD et al.: Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res 2012; 72(19): 5004-5013.
33. Fakhry C, Westra WH, Li S et al.: Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008; 100(4): 261-269.
34. Rischin D, Young RJ, Fisher R et al.: Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol 2010; 28: 4142-4148.
35. Rich JT, Milov S, Lewis JS et al.: Transoral laser microsurgery (TLM) +/- adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope 2009; 119: 1709-1719.
36. National Comprehensive Cancer Network. Head and neck cancers; http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed 15.03.2017).
37. Ang KK, Harris J, Wheeler R et al.: Human papillomavirus and survival of patients with oropharyngeal cancer. NEJM 2010; 363: 24-35.
38. Lydiatt WM, Ridge JA, Patel SG et al.: Oropharynx (p16-) and Hypopharynx. In: AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017. 123.
39. O’Sullivan B, Lydiatt WM, Haughey BH et al.: HPV-Mediated (p16+) Oropharyngeal Cancer. In: AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017. 123.
40. Helwick C: HPV-Positive Head and Neck Cancer: When Can Chemotherapy Be Omitted? The Asco Post; http://www.ascopost.com/issues/september-25-2015/hpv-positive-head-and-neck-cancer-when-can-chemotherapy-be-omitted/ (accessed 21.08.2017).
41. Marur S, Li S, Cmelak AJ et al.: E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx – ECOG-ACRIN Cancer Research Group. J Clin Oncol 2017: 35: 490-497.
42. NRG-HN002: A randomized phase II trial for patients with p16 positive, non-smoking associated, locoregionally advanced oropharyngeal cancer; https://www.nrgoncology.org/Clinical-Trials/NRG-HN002.
