Magdalena Świątkowska1, *Paula Piekoszewska-Ziętek1, Dariusz Gozdowski2, Dorota Olczak-Kowalczyk1
The usefulness of Spotchem® Analyser (Arkray) in determining the risk in oral diseases in adolescents – a pilot study
Ocena przydatności analizatora Spotchem® (Arkray) w diagnostyce ryzyka chorób jamy ustnej u dzieci. Badanie pilotażowe
1Department of Paediatric Dentistry, Medical University of Warsaw
Head of Department: Professor Dorota Olczak-Kowalczyk, MD, PhD
2Department of Experimental Design and Bioinformatics, Warsaw University of Life Sciences
Head of Department: Krzysztof Pawłowski, PhD
Streszczenie
Wstęp. Od dawna stosowanym testem oceny parametrów fizykochemicznych śliny jest test Saliva Check Buffer®, a poziomu bakterii kariogennych – CRT Bacteria®. Nowym urządzeniem oceny parametrów śliny jest analizator Spotchem®.
Cel pracy. Określenie zgodności wyników badań uzyskiwanych analizatorem Spotchem® i testami Saliva Check Buffer® i CRT Bacteria® oraz ocena korelacji uzyskanych wyników ze stanem higieny jamy ustnej, dziąseł i uzębienia w diagnostyce ryzyka chorób jamy ustnej u dzieci.
Materiał i metody. U pacjentów w wieku 12-17 lat oceniono: stan higieny jamy ustnej (%API; OHI), dziąseł (GI) i uzębienia (ICDAS II) oraz wykonano badania testami Saliva Check Buffer® (GC) i CRT Bacteria® (Ivoclar Vivadent) oraz analizatorem Spotchem® (Arkray). Uzyskano zgodę komisji bioetycznej Warszawskiego Uniwersytetu Medycznego oraz pisemne zgody rodziców lub opiekunów prawnych pacjentów.
Wyniki. Zbadano 25 pacjentów (średnia wieku 13,7 ± 2,2 roku). Średnie wartości wskaźników wynosiły: OHI-S – 0,93 ± 0,43; API% – 72 ± 0,26; GI – 0,83 ± 0,61; PUWZ – 6,44 ± 4,12. Aktywne plamy próchnicowe występowały u 13 pacjentów (średnio 2,2 ± 2,92 plamy). Analiza Spearmana wykazała istotne związki pH w teście Saliva Check Buffer® i zdolności buforowej śliny (r = 0,608), jej kwasowości (r = -0,713) w badaniu Spotchem® oraz ujemną korelację buforowości i pH śliny w teście Spotchem® (r = -0,845). Wysoka liczebność bakterii Streptococcus mutans (> 105 CFU/ml) oceniana testem CRT Bacteria® istotnie korelowała z wysokim poziomem aktywności metabolicznej bakterii w badaniu analizatorem Spotchem® (r = 0,54). Potwierdzono istotne korelacje następujących parametrów: OHI i wysoka (r = 0,46) oraz średnia (r = -0,54) aktywność metaboliczna S. mutans; GI i poziom białka w ślinie (r = 0,42); obecność ubytków próchnicowych i poziom białek (r = 0,40), erytrocytów w ślinie (r = 0,47) (Spotchem®) oraz liczebność S. mutans i Lactobacillus spp. (CRT Bacteria®) (r odpowiednio 0,47 i 0,42).
Wnioski. Wyniki badania śliny analizatorem Spotchem® są skorelowane z uzyskiwanymi w znanych testach ślinowych oraz z parametrami zdrowia jamy ustnej. Jego przydatność kliniczna powinna być potwierdzona dalszymi badaniami.
Summary
Introduction. Saliva Check Buffer® has been long used for assessing saliva physical and chemical properties, while CRT Bacteria® is used for estimating the count of cariogenic bacteria. Spotchem® Analyser is a new device for the assessment of saliva properties.
Aim. To determine the consistency of results obtained using Spotchem®, Saliva Check Buffer® and CRT Bacteria® kits, as well as to evaluate the correlations between the obtained results and oral hygiene status, ICDAS II indices in the diagnosis of the risk of oral diseases in children.
Material and methods. Patients aged 12-17 years were evaluated for oral hygiene (% API, OHI), gingival inflammation (GI), and carious lesions (ICDAS II). Salivary tests using Saliva Check Buffer® (GC), CRT Bacteria® (Ivoclar Vivadent) and Spotchem® (Arkray) Analyser were conducted. The consent of the bioethical committee of Warsaw Medical University, as well as written consent from all the parents of all the subjects or legal guardians of all the subjects were obtained.
Results. The study included 25 patients (mean age 13.7 ± 2.2 years). The following mean index values were obtained: OHI-S – 0.93 ± 0.43; API% – 72 ± 0.26; GI – 0.83 ± 0.61; DMFt – 6.44 ± 4.12. Active white lesions were observed in 13 patients (mean number of lesions 2.2 ± 2.92). Spearman’s rank correlation coefficient showed significant correlations between pH values according to Saliva Check Buffer® and salivary buffer capacity (r = 0.608) and acidity (r = -0.713) according to Spotchem®; as well as a negative correlation between salivary buffer capacity and pH values in Spotchem® (r = -0.845). High count of S. mutans (> 105 CFU/mL) assessed by CRT Bacteria® correlated significantly with high bacteria count estimated by the Spotchem® Analyser (r = 0.54). Significant correlations were found between OHI and high (r = 0.46) and average (r = -0.54) metabolic activity of S. mutans; GI and salivary protein levels (r = 0.42); carious lesions and salivary protein levels (r = 0.40); salivary blood levels (r = 0.47) (Spotchem®) and the levels of S. mutans and Lactobacillus spp. (CRT Bacteria®) (0.47 and 0.42, respectively).
Conclusions. The parameters estimated by the Spotchem® Analyser were correlated with the results obtained with the commonly known salivary kits and oral health indices. However, its clinical relevance should be confirmed by further studies.

Introduction
Contemporary dental treatment involves risk assessment and monitoring for oral diseases, dental caries and periodontal diseases in particular (1, 2). Risk assessment for these diseases requires the determination of the balance between pathogenic factors and body defences, including salivary physicochemical properties and oral microbial load (3-6).
Commercially available saliva tests with proven clinical usefulness, such as Saliva Check Buffer®, CRT Baceria® (Ivoclar Vivadent), Dentocult SM and Dentobuff (Orion Diagnostica), have been long used in the clinical practice. Saliva Buffer Check® allows an assessment of salivary pH and buffer capacity. CRT Bacteria® is used to determine the levels of Streptococcus mutans and Lactobacillus spp. following stimulated saliva application in SM and LAB-AGAR culture medium and incubation at 37°C for 48 hours. Patients with bacterial count > 105 CFU/mL are considered to be at an increased risk of dental caries (5, 7, 8).
Spotchem® (Arkray) is a novel test that allows an assessment of salivary S. mutans count, acidity, and buffer capacity, as well as protein, blood, leukocyte and ammonia levels. The scale ranges between 0 and 100 units for each test. The analyser measures the following parameters: S. mutans levels (106-108 CFU/mL); acidity (pH 6.0-8.0); salivary buffer capacity (pH 2.8-6.0); salivary blood levels (0-0.50 mg/dL); salivary leukocyte count (0-200 U/L); salivary protein levels (0-6 mg/dL); and salivary ammonia levels (0-10 000 N-μg/dL). Mean values are as follows: 28-47 units for S. mutans levels; 35-52 for acidity; 28-47 for salivary buffer capacity; 14-29 for salivary blood levels; 37-60 for salivary leukocyte count; 36-53 for salivary protein levels; 43-83 for salivary ammonia levels.
So far, no studies using this device have been published.
Aim
The aim of the study was to determine the consistency of results using Spotchem®, Saliva Check Buffer® and CRT Bacteria® kits, as well as to assess correlations between the obtained results and oral hygiene status (including gingival and dental health status) in the diagnosis of the risk of oral diseases in paediatric patients.
Material and methods
Generally healthy patients with permanent dentition aged between 12 and 17 years, who reported for dental visits to the Department of Paediatric Dentistry of the Medical University of Warsaw, were included in the study. A written consent to participate in the study and patient’s cooperation were inclusion criteria. Patients using orthodontic appliances, as well as patients with a medical history of chronic diseases or pharmacotherapy affecting salivary properties were excluded from the study. This pilot study has been approved by the Bioethical Committee of the Medical University of Warsaw, no. of approval KB/6/2017. Written consent of the parents or guardians of the patients were obtained. The study involved clinical evaluation and assessment of salivary parameters using commercially available tests. Clinical evaluation was performed in a dental office setting to assess oral health (Approximal Plaque Index %API according to Lange et al., the Simplified Oral Hygiene Index OHI-S) (9, 10), gingival health (GI by Silness & Löe) (11, 12), and dental health – the presence of carious lesions (International Caries Detection and Assessment System II – ICDAS II) (13). Salivary properties were assessed using commercially available tests, such as Saliva Check Buffer® (GC) and CRT Bacteria® (Ivoclar Vivadent), in accordance with manufacturers’ instructions, as well as Spotchem® Analyser (Arkray). Saliva Check Buffer® (GC) and CRT Bacteria® (Ivoclar Vivadent) were used to assess parameters measured by Spotchem®. The tests were performed 30 minutes before the use of analyser. Both stimulated and unstimulated saliva (according to manufacturer’s instructions) was tested in the morning hours, at least 2 hours after last meal or tooth brushing. During the test with the Spotchem® Analyser, the patient received a rinse solution for a 10-second mouthwash, after which all of the liquid was spat into a disposable cup. Using a pipette, the spat liquid was applied on a test strip, which was placed in the analyser connected to the computer. After 260 seconds, the computer programme produced results for the metabolic activity of S. mutans, acidity, salivary buffer capacity, protein, blood, leukocyte and ammonia levels (fig. 1).
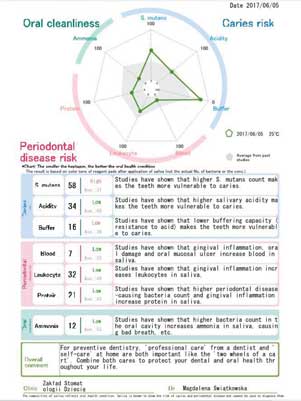
Fig. 1. An exemplary form with results obtained in the Spotchem® Analyser
Saliva Check Buffer® was used to assess unstimulated salivary pH and stimulated salivary buffer capacity. Unstimulated salivary pH was considered normal for 6.8-7.8; moderately acidic for 6.0-6.6, and acidic for 5.0-5.8. Salivary buffer capacity was assessed after stimulated saliva collection by the patient during a 5-minute paraffin chewing. Using a colour score (green – 4 points, green/blue – 3 points, blue – 2 points, blue/red – 1 point and red – 0 points), the parameter was ranked as high (10-12 points), average (6-9 points) or low (0-5). CRT Bacteria® was used to evaluate S. mutans counts (the result was recorded after 48 hours of incubation at 37°C). The assessment was performed based on the number of bacterial colonies on the agar. Patients with bacterial counts > 105 CFU/mL were considered to be at a high risk of dental caries.
Correlations between the analysed variables were assessed based on Spearman’s rank correlation coefficient. Additionally, a simple linear regression analysis or logistic regression was used to evaluate selected correlations. Statistica 8 used for statistical analysis and a p ≤ 0.05 was considered statistically significant.
Results
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
29 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
69 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
129 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 78 zł
Piśmiennictwo
1. Turska-Szybka A, Grudziąż-Sękowska J, Olczak-Kowalczyk D: Czynniki ryzyka próchnicy wczesnego dzieciństwa i indywidualna ocena poziomu ryzyka na podstawie CAMBRA. Nowa Stomatol 2011; 3: 119-127.
2. American Academy of Pediatric Dentistry: Guideline on caries-risk assessment and management for infants, children, and adolescents. Pediatr Dent 2013; 35(5): 157-164.
3. Ferreira-Nóbilo NP, Tabchoury CP, Sousa Mda L, Cury JA: Knowledge of dental caries and salivary factors related to the disease: influence of the teaching-learning process. Braz Oral Res 2015; 29(1): 1-7.
4. Leone CW, Oppenheim FG: Physical and chemical aspects of saliva as indicators of risk for dental caries in humans. J Dent Educ 2001; 65(10): 1054-1062.
5. Wongkamhaeng K, Poachanukoon O, Koontongkaew S: Dental caries, cariogenic microorganisms and salivary properties of allergic rhinitis children. Int J Pediatr Otorhinolaryngol 2014; 78(5): 860-865.
6. Zabokova-Bilbilova E, Sotirovska-Ivkovska A, Georgiev Z, Stefanovska E: Evaluation of buffer capacity of saliva in caries-free and caries-active children. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2013; 34(2): 151-157.
7. Gilbert K, Joseph R, Vo A et al.: Children with severe early childhood caries: streptococci genetic strains within carious and white spot lesions. J Oral Microbiol 2014; 6(1): 1-11.
8. Twetman S, Fritzon B, Jensen B et al.: Pre- and post-treatment levels of salivary mutans streptococci and lactobacilli in pre-school children. Int J Paediatr Dent 1999; 9(2): 93-98.
9. Lange DE, Plagmann HC, Eenboom A, Promsberger A: Klinische Bewertungsverfahren zur Objektivierung der Mundhygiene. Dtsch. Zahnärztl Z 1977; 32: 44-47.
10. Greene JC, Vermillion JR: The simplified oral hygiene index. JADA 1964; 68(1): 7-13.
11. Silness J, Löe H: Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 1964; 22: 121-135.
12. Löe H: The gingival index, the plaque index and the retention index systems. J Periodontol 1967; 38 (suppl.): 610.
13. International Caries Detection and Assessment System (ICDAS) Coordinating Committee (2005) Criteria Manual: International Caries Detection and Assessment System (ICDAS II). www.icdas.org (data dostępu: 15.11.2008).
14. Animireddy D, Reddy Bekkem VT, Vallala P et al.: Evaluation of pH, buffering capacity, viscosity and flow rate levels of saliva in caries-free, minimal caries and nursing caries children: An in vivo study. Contemp Clin Dent 2014; 5(3): 324-328.
15. Kaur A, Kwatra KS, Kamboj P: Evaluation of non-microbial salivary caries activity parameters and salivary biochemical indicators in predicting dental caries. J Indian Soc Pedod Prev Dent 2012; 30(3): 212-217.
16. Singh S, Sharma A, Sood PB et al.: Saliva as a prediction tool for dental caries: an in vivo study. J Oral Biol Craniofac Res 2015; 5(2): 59-64.
17. Kuriakose S, Sundaresan C, Mathai V et al.: A comparative study of salivary buffering capacity, flow rate, resting pH, and salivary Immunoglobulin A in children with rampant caries and caries-resistant children. J Indian Soc Pedod Prev Dent 2013; 31(2): 69-73.
18. Makawi Y, El-Masry E, El-Din HM: Salivary carbonic anhydrase, pH and phosphate buffer concentrations as potential biomarkers of caries risk in children. J Unexplored Med Data 2017; 2: 9-15.
19. Bagherian A, Asadikaram G: Comparison of some salivary characteristics between children with and without early childhood caries. Indian J Dent Res 2012; 23(5): 628-632.
20. Prabhakar AS, Reshma D, Raju OS: Evaluation of flow rate, pH, buffering capacity, calcium, total protein and total antioxidant levels of saliva in caries free and caries active children – an in vivo study. Int J Clin Pediatr Dent 2009; 2(1): 9-12.
21. Preethi BP, Reshma D, Anand P: Evaluation of flow rate, pH, buffering capacity, calcium, total proteins and total antioxidant capacity levels of saliva in caries free and caries active children: an in vivo study. IJCB 2010; 25(4): 425-428.
22. Piróg A, Kalińska A, Gozdrowski D, Olczak-Kowalczyk D: Wpływ parametrów fizykochemicznych śliny na stan uzębienia, dziąseł i błony śluzowej jamy ustnej u dzieci zdrowych. J Stomatol 2013; 66(2): 154-169.
23. Malekipour MR, Messripour M, Shirani F: Buffering capacity of saliva in patients with active dental caries. Asian J Biochem 2008; 3: 280-283.
24. Sakeenabi B, Hiremath SS: Dental caries experience and salivary Streptococcus mutans, lactobacilli scores, salivary flow rate, and salivary buffering capacity among 6-year-old Indian school children. J Int Soc Prev Community Dent 2011; 1(2): 45-51.
25. Gamboa F, Estupifian M, Galindo A: Presence of Streptococcus mutans in saliva and its relationship with dental caries: Antimicrobial susceptibility of the isolates. Universitas Scientiarum 2004; 9: 23-27.
26. Xu H, Hao W, Zhou Q et al.: Plaque bacterial microbiome diversity in children younger than 30 months with or without caries prior to eruption of second primary molars. PLoS One 2014; 9(2): e89269.
27. Tao Y, Zhou Y, Ouyang Y, Lin H: Dynamics of oral microbial community profiling during severe early childhood caries development monitored by PCR-DGGE. Arch Oral Biol 2013; 58(9): 1129-1138.
28. Jiang S, Jin L, Lo ECM: Salivary microbiome diversity in caries-free and caries-affected children. Int J Mol Sci 2016; 17(12): 1978.
29. Hallett K, O’Rourke P: Baseline dental plaque activity, mutans streptococci culture, and future caries experience in children. Pediatr Dent 2013; 35(7): 523-528.
30. Bendoraitienė E, Z?bienė J, Vasiliauskienė I et al.: Periodontal status in 18-year-old Lithuanian adolescents: an epidemiological study. Medicina 2017; 53(4): 253-258.
31. Fábián TK, Hermann P, Beck A et al.: Salivary Defense Proteins: Their network and role in innate and acquired oral immunity. Int J Mol Sci 2012; 13(4): 4295-4320.
32. Roa NS, Chaves M, Gómez M, Jaramillo LM: Association of salivary proteins with dental caries in a Colombian population. Acta Odontol Latinoam 2008; 21(1): 69-75.
33. Bhalla S, Tandon S, Satyamoorthy K: Salivary proteins and early childhood caries: A gel electrophoretic analysis. Contemp Clin Dent 2010; 1(1): 17-22.
34. Shu M, Morou-Bermudez E, Suárez-Pèrez E et al.: The relationship between dental caries status and dental plaque urease activity. Oral Microbiol Immunol 2007; 22(1): 61-66.
35. Nascimento MM, Gordan VV, Garvan CW et al.: Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol 2009; 24(2): 89-95.
36. Morou-Bermudez E, Elias-Boneta A, Billings RJ et al.: Urease activity in dental plaque and saliva of children during a three-year study period and its relationship with other caries risk factors. Arch Oral Biol 2011; 56(11): 1282-1289.
37. Toro E, Nascimento MM, Suarez-Perez E et al.: The effect of sucrose on plaque and saliva urease levels in vivo. Arch Oral Biol 2010; 55(3): 249-254.

