© Borgis - Postępy Nauk Medycznych 4/2014, s. 238-244
*Magdalena Ćwiklińska1, Walentyna Balwierz1, Mirosław Bik-Multanowski2, Tomasz Klekawka1
Polimorfizm 677C>T genu reduktazy 5,10-metylenotetrahydrofolianowej (MTHFR) a wczesne powikłania leczenia wysokimi dawkami metotreksatu u dzieci z ostrą białaczką limfoblastyczną
677C>T 5,10-methylenetetrahydrofolate reductase reductase (MTHFR) polymorphism and early toxicity of high-dose methotrexate in children treated for acute lymphoblastic leukemia
1Department of Pediatric Oncology and Hematology, Polish American Institute of Pediatrics, Jagiellonian University Medical College, Kraków
Head of Department: prof. Walentyna Balwierz, MD, PhD
2Chair and Department of Pediatrics, Polish American Institute of Pediatrics, Jagiellonian University Medical College, Kraków
Head of Chair and Department: prof. Jacek J. Pietrzyk, MD, PhD
Streszczenie
Wstęp. Wysokie dawki metotreksatu (HD-Mtx) pomimo monitorowania eliminacji leku i podaży leukoworyny u niektórych pacjentów wywołują nasilone objawy toksyczne. Jednym z kluczowych enzymów szlaku przemian folianów, występującym w zmiennej, zależnej od polimorfizmów genowych aktywności, którego działanie zaburza Mtx, jest reduktaza metylenotetrahydrofolianowa (MTHFR).
Cel pracy. Analiza występowania i nasilenia wczesnych toksyczności terapii HD-Mtx u dzieci leczonych z powodu ALL, w zależności od polimorfizmu genowego 677C>T MTHFR.
Materiał i metody. Badaniem retrospektywnym objęto 160 dzieci w wieku 1-17 lat, leczonych w jednym ośrodku onkologii dziecięcej w latach 1993-2007 zgodnie ze zmodyfikowanym protokołem ALL-BFM-90 i ALL-IC-2002. Nosicielstwo polimorfizmu 677C>T MTHFR oceniono techniką PCR-RFLP, a parametry farmakokinetyki Mtx metodą immunoenzymatyczną. Bliskie toksyczności łącznie 617 cykli chemioterapii z zastosowaniem dawki 2 g/m2 i 3 g/m2 Mtx analizowano zgodnie ze skalą NCI-CTC.
Wyniki. W badanej grupie pacjenci o „dzikim” genotypie CC, heterozygoty CT i homozygoty TT stanowili odpowiednio: 42,5; 48,75 i 8,75%. Nudności i wymioty częściej występowały u heterozygot CT (p = 0,03). U pacjentów TT odnotowano częstsze: opóźnienia w kontynuacji leczenia (p = 0,01), małopłytkowość (p = 0,03) i ostre objawy neurotoksyczne (p = 0,05). Wyższe stopnie toksyczności wątrobowych, nudności, zapalenia śluzówek jamy ustnej i zakażeń obserwowano u homozygot TT. Ostra niewydolność nerek o nieznanej etiopatogenezie wystąpiła u dwóch pacjentów: heterozygoty CT i homozygoty TT.
Wnioski. Uzyskane wyniki wskazują na związek allela T z większym nasileniem ostrych toksyczności terapii HD-Mtx u dzieci. Celowa jest kontynuacja badań z udziałem dużych grup pacjentów i uwzględnieniem także innych istotnych polimorfizmów genowych.
Summary
Introduction. High doses of methotrexate (HD-Mtx) in some patients may cause severe adverse effects despite monitoring of Mtx elimination and administering of calcium folinate.
One of the most important enzymes of the folate metabolic pathway affected by Mtx is methylenetetrahydrofolate reductase (MTHFR) which activity depends on genetic polymorphisms.
Aim. Analysis of occurrence and intensity of Mtx therapy related early toxicities in children treated because of acute lymphoblastic leukemia (ALL) in dependency on 677C>T MTHFR gene polymorphism.
Material and methods. One hundred and sixty children (age: 1-17 years) treated in one pediatric oncology department between 1993 and 2007 according to ALL-BFM-90 and ALL-IC-2002 protocols were included into retrospective study. The presence of 677C>T MTHFR polymorphism was evaluated with the use of PCR-RFLP technique, and Mtx pharmacokinetics parameters were evaluated with immunoenzymatic method. Early toxicities of 617 chemotherapy cycles (Mtx dose 2 g/m2 and 3 g/m2) were analyzed according to NCI-CTC scale.
Results. In analyzed group the patients with wild-type genotype CC, heterozygotes CT and homozygotes TT were 42.5, 48.75 and 8.75%, accordingly. Nausea and vomiting were significantly more common in CT heterozygotes (p = 0.03). In TT patients delays of therapy (p = 0.01), thrombocytopenia (p = 0.03) and acute neurotoxicities (p = 0.05) were more common.
More severe liver toxicities, nausea, stomatitis, and infections were observed in TT homozygotes. Acute renal insufficiency of unknown etiology occurred in two patients (CT heterozygote and TT homozygote).
Conclusions. Obtained results indicate the possible correlation between the presence of T allel and high risk of acute toxicities of HD-Mtx therapy in children. It is necessary to continue studies including higher number of patients with evaluation of other important genetic polymorphisms.

Introduction
Acute lymphoblastic leukemia (ALL) is a most frequent neoplasm of childhood. With use of multimodal, multidrug therapy it can be cured in about 80% of cases. Standard chemotherapy is expected to result in toxicities that rarely are considered as life-threatening ones but in most of the patients these toxicities can be strongly pronounced and thus constitute a reason for therapy delay what increases a risk of leukemia relapse. Acute toxicities are particularly frequent in case of high doses of cytotoxic drugs used in repeated cycles as for example in case of high dose methotrexate (HD-Mtx) administration in remission consolidation phase in ALL (1-4). This cytostatic agent belongs to the antimetabolite group of cytostatics. It’s action comprises blockage of several enzymes involved in folate metabolism pathway what results in block in purine synthesis, protein methylation inhibition and i.a. transformation of homocysteine to methionine (3, 5-9). Inhibition of cell divisions resulting from disturbed DNA synthesis and repair acts against the leukemic cell clone but also injuries healthy tissues, especially these that undergo rapid cell division as epithelial cells, mucosae, and bone marrow. Most frequently observed methotrexate toxicities are: myelosuppression, mucositis, skin changes, liver and kidney dysfunction, acute neurological toxicities and also infections. Life-threatening toxicities of high-dose methotrexate administration are however relatively rare, of which most important one is kidney failure. Prevalence and degree of observed toxic side effects of methotrexate results mainly from prolonged methotrexate exposition as well as individual patient’s susceptibility to that drug. Gene polymorphisms resulting in modulation of enzyme activity that results in important metabolic pathways modification has been widely studied lately. Certain gene polymorphisms can influence not only the effectiveness of anti-cancer therapy and risk of relapse but also the occurrence and intensity of chemotherapy side effects (2-6, 8-14). In case of folate metabolic pathways that are influenced by methotrexate, methylenetetrahydrofolate reductase (MTHFR) activity is of special meaning (MTHFR). This enzyme is involved in the equilibrium of protein methylation processes (including homocysteine homeostasis) and urine and thymidine synthesis (DNA regeneration) as well. Among abundantly described MTHFR gene polymorphisms, the commonly observed 677C>T polymorphism is of special relevance as it results in significant decrease of MTHFR enzyme activity (10, 11). The prevalence of polymorphic T allel varies between different geographical regions that i.a. are known to differ in nutritional habits. This specific allel is most frequently observed in mediterranean and central-African population where diet is generally considered as folate-rich one. In Caucasian population MTHFR activity is observed in about half of population as the T allel is present in 40% of this population in its heterozygotic CT form and in about 10% of population in homozygotic TT form (5, 7, 10, 11, 15, 16). As a result of heterozygotic CT form occurrence and even more in case of 677 nucleotide TT homozygosity a thermolabile enzyme of decreased activity is produced (60 and 30% of activity, respectively). This can influence the individual patient’s methotrexate sensitivity. Numerous published papers indicate a relationship between the presence of 677C>T MTHFR gene polymorphism and the observed intensity of acute methotrexate toxicities both in case of low as well as high methotrexate doses use (1, 9, 10, 17-20). Some papers indicate a relationship between this gene polymorphism and worse treatment results in children treated for ALL. These treatment failures may result not only from deaths of therapy toxicities (influenced i.a. by endothelium dysfunction secondary to hiperhomocysteinemia) but also an increased risk of relapse in T allel carriers in whom DNS synthesis suppression is decreased (1, 2, 21).
Aim
Aim of present paper is analysis of high-dose methotrexate acute toxicities frequency and intensity in children treated for ALL depending on 677C>T MTHFR gene polymorphism presence.
Material and methods
Retrospective analysis of 160 children with ALL (age: 1-17 years), treated from 1993 to 2007 at Pediatric Oncology and Hematology Department, Polish-American Institute of Pediatrics, Jagiellonian University Medical College in Kraków with high-dose methotrexate as a consolidation ALL therapy (doses: 2 g/m2 – modified ALL-BFM-90 protocol and 3 g/m2 – ALL-IC-BFM-2002 protocol) was performed. Therapy inclusion criteria comprised: good general status of the patient, no evidence for acute infections, normal liver and kidney function and elimination of drugs known to interfere with methotrexate metabolism. Therapy was administered four times, every two weeks. Patients were initially hydrated and received proper alkalization prior to 24-hour continuous methotrexate infusion administered with use of volumetric pump and central venous access. A lumbar puncture with intrathecal methotrexate or methotrexate/cytarabine/hydrocortisone administration (in case of 3 g/m2 and 2 g/m2 methotrexate dose respectively) was performed within two hours from the beginning of methotrexate infusion. Patients also received oral mercaptopurine (25 mg/m2) daily. A specific antidote – leucovorin (15 mg/m2) was administered in 42, 48 and 54 hour from the start of methotrexate infusion. Methotrexate serum concentration was routinely assessed in 24, 36, 42 and 48 hour form start of methotrexate infusion (EMIT® – Syva immunoenzymatic method, with use of VIVA-Vitalab analyzer, DADE-BEHRING, USA). Methotrexate serum concentrations obtained after completion of methotrexate infusion were considered as representative for steady state. Hour 48 serum methotrexate concentration exceeding critical values (0.40 μmol/l) resulted in leucovorin dose escalation, hydration intensification and in prolonged methotrexate serum concentration until safe values were reached (< 0.25 μmol/l) in each case. Methotrexate elimination pharmacokinetics were assessed with use of first degre elimination constants for both phases of methotrexate elimination estimated with Wagner’s formula (Kel = lnc1 – lnc2/t2 – t1).
Presence of 677C>T MTHFR gene polymorphism was identified in DNA isolated from peripheral blood leukocytes with use of PCR-RFLP technique. Starter sequences used in DNA amplification reaction were: CTG ACC TGA AGC ACT TGA AGG (forward) and AGT GAT GCC CAT GTC GGT (reverse). PCR reaction products were processed with restriction enzyme Hinfl1, which detects the locus of cytosine to thymidine substitution in 677th nucleotide. Obtained DNA fragments were separated with use of agarose gel electrophoresis (fig. 1).
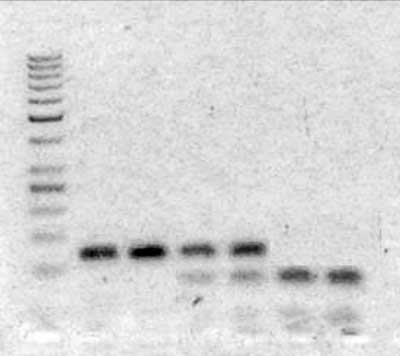
Fig. 1. Result of agarose gel electrophoresis of DNA samples treated with Hinfl1 enzyme – 677C>T MTHFR gene polymorphism. From left to right: ladder, two samples – CC homozygotes (122bp DNA fragments), two samples – CT heterozygotes (two DNA fragments: 122bp – 677C allel and two sequences of 82bp – 677T allel), two samples – TT homozygotes (DNA fragments of 82bp corresponding to 677T allel with Hinfl1restriction site).
National Cancer Institute Common-Toxicity-Criteria in GPOH modification (German Society of Pediatric Oncology/Hematology) (22) was used to assess organ and systemic toxicities observed during the high-dose methotrexate treatment.
An analysis of total of 617 chemotherapy cycles with use of 2 g/m2 and 3 g/m2 methotrexate doses was performed. Statistical analyses were performed with use of STATISTICA software. Chi-square test and Fisher exact test were used to identify relations between qualitative features. Consistency of allele separation within observed group of patients with expected allele distribution according to Hardy-Weinberg’s rule was checked with use of the Chi-square test. One-sided fraction test was applied to check the influence of 677C>T MTHFR gene polymorphism on the incidence of acute HD-Mtx therapy toxicities. Multiple logistic regression analysis was performed to identify risk factors of increased HD-Mtx therapy toxicities. Statistical results were verified at the significance level of p = 0.05.
Results
Among 160 analyzed cases treated with HD-Mtx, 78 (48.75%) were CT heterozygotes, 68 (42.5%) were identifies as a wild-type CC gene carriers and 14 (8.75%) were TT homozygotes. The distribution of 677C>T MTHFR gene polymorphisms within analyze group was consistent with the Hardy-Weinberg equation conditions (χ2 = 0.323; p = 0.57).
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
29 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
69 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
129 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 78 zł
Piśmiennictwo
1. El-Khodary NM, El-Haggar SM, Eid MA et al.: Study of the pharmacokinetic and pharmacogenetic contribution to the toxicity of high-dose methotrexate in children with acute lymphoblastic leukemia. Med Oncl 2012; 29: 2053-2062.
2. Ongaro A, De Mattei M, Della Porta MG et al.: Gene polymorphisms in folate metabolizing enzymes in adult acute lymphoblastic leukemia: effects on methotrexate-related toxicity and survival. Haematologica 2009; 94: 1391-1398.
3. Salazar J, Altès A, del Rio E et al.: Methotrexate consolidation treatment according to pharmacogenetics of MTHFR ameliorates event-free survival in childhood acute lymphoblastic leukaemia. The Pharmacogenomics J 2012; 12: 379-385.
4. Kotnik BF, Grabnar I, Grabar PB et al.: Association of genetic polymorphism in folate metabolic pathway with methotrexate pharmacokinetics and toxicity in childhood acute lymphoblastic leukemia and malignant lymphoma. Eur J Clin Pharmacol 2011; 67: 993-1006.
5. Aplenc R, Thompson J, Han P et al.: Methylenetetrahydrofolate reductase polymorphisms and therapy response in pediatric acute lymphoblastic leukemia. Cancer Research 2005; 65: 2482-2487.
6. de Jonge R, Hooijberg JH, van Zelst BD et al.: Effect of polymorphisms in folate-related genes on in vitro methotrexate sensivity in pediatric acute lymphoblastic leukemia. Blood 2005; 106: 717-720.
7. Kishi S, Griener J, Cheng C et al.: Homocysteine, pharmacogenetic, and neurotoxicity in children with leukemia. J Clin Oncol 2003; 21: 3084-3091.
8. Moe PJ, Holen A: High-dose methotrexate in childhood ALL. Pediatr Hematol Oncol 2000; 17: 615-622.
9. Pui CH, Relling MV, Evans WE: Role of pharmacogenetics and pharmacodynamics in the treatment of acute lymphoblastic leukemia. Best Pract Res Clin Haematol 2002; 15: 741-756.
10. Costea I, Morghrabi A, Laverdiere C et al.: Folate cycle gene variants and chemotherapy toxicity in patients with acute lymphoblastic leukemia. Haematologica 2006; 91: 1113-1116.
11. De Mattia E, Toffoli G: C677T and A1298C MTHFR polymorphisms, a challenge for antifolate and fluoropyrimidine-based therapy personalisation. Eur J of Cancer 2009; 45: 1333-1351.
12. Radtke S, Zolk O, Renner B et al.: Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity and outcome in childhood acute lymphoblastic leukemia. Blood 2013; 1: 1-23.
13. Erčulj N, Faganel Kotnik B, Debeljak M et al.: Influence of folate pathway polymorphisms on high-dose methotrexate-related toxicity and survival in childhood acute lymphoblastic leukemia. Leuk Lymphoma 2012; 53: 1096-1104.
14. Gervasini G, Vagace JM: Impact of genetic polymorphisms on chemotherapy toxicity in childhood acute lymphoblastic leukemia. Front In Gen 2012; 3: 1-11.
15. Aplenc R, Lange B: Pharmacogenetic determinants if outcome in acute lymphoblastic leukemia. Br J Haematol 2004; 125: 421-434.
16. Chiusolo P, Reddiconto G, Farina G et al.: MTHFR polymorphisms’ influence on outcome and toxicity in acute lymphoblastic leukemia patients. Leukemia Research 2007; 31: 1669-1674.
17. Liu SG, Li ZG, Gao C et al.: Effects of methylenetetrahydrofolate reductase gene polymorphisms on toxicities during consolidation therapy in pediatric acute lymphoblastic leukemia in a Chinese population. Leuk Lymphoma 2011; 52: 1030-1040.
18. Taub JW, Matherly LH, Ravindranath Y et al.: Polymorphisms in methylenetetrahydrofolate reductase and methotrexate sensivity in childhood acute lymphoblastic leukemia. Leukemia 2002; 16: 764-765.
19. Ulrich CM, Yasui Y, Storb R et al.: Pharmacogenetics of methotrexate: toxicity among marrow transplantation patients varies with the methylenetetrahydrofolate reductase C677T polymorphism. Blood 2001; 98: 321-324.
20. Weisman MH, Furst DE, Park GS et al.: Risk genotypes in folate-dependent enzymes and their association with methotrexate-related side effects in rheumatoid arthritis. Arthritis and Rheumatism 2006; 54: 607-612.
21. Pietrzyk JJ, Bik-Multanowski M, Balwierz W et al.: Additional genetic risk factor for death in children with acute lymphoblastic leukemia: a common polymorphism of the MTHFR gene. Pediatr Blood & Cancer 2009; 52: 364-368.
22. www.accessdata.fda.gov/scripts/cder/onctools/toxcrit1.cfm.
23. Chiusolo P, Giammarco S, Bellesi S et al.: The role of MTHFR and RFC1 polymorphisms on toxicity and outcome of adult patients with hematological malignancies treated with high-dose methotrexate followed by leucovorin rescue. Cancer Chemother Pharmacol 2012; 69: 691-696.
24. Toffoli G, Russo A, Innocenti F et al.: Effect of methylenetetrahydrofolate reductase 677C>T polymorphism on toxicity and homocysteine plasma level after chronic methotrexate treatment of ovarian cancer patients. Int J Cancer 2003; 103: 294-299.
25. Kim I, Lee KH, Kim JH et al.: Polymorphisms of the methylenetetrahydrofolate reductase gene and clinical outcomes in HLA-matched sibling allogeneic hematopoietic stem cell transplantation. Annals of Hematol 2007; 86: 41-48.
26. Shimasaki N, Mori T, Torii C et al.: Influence of MTHFR and RFC1 polymorphisms on toxicities during maintenance chemotherapy for childhood acute lymphoblastic leukemia or lymphoma. J Pediatr Hematol Oncol 2008; 30: 347-352.
27. Kantar M, Kosova B, Cetingul N et al.: Methylenetetrahydrofolate reductase C677T and A1298C gene polymorphisms and therapy-related toxicity in children treated for acute leukemia and non-Hodgkin lymphoma. Leuk Lymphoma 2009; 50: 912-917.
28. Seidemann K, Book M, Zimmermann M et al.: MTHFR 677C>T polymorphism is not relevant for prognosis or therapy-associated toxicity in pediatric NHL: results from 484 patients of multicenter trial NHL-BFM 95. Ann Hematol 2006; 85: 291-300.
29. Shimasaki N, Mori T, Samejima H et al.: Effects of methylenetetrahydrofolate reductase and reduced folate carrier polymorphisms on high-dose methotrexate-induced toxicities in children with acute lymphoblastic leukemia or lymphoma. J Pediatr Hematol Oncol 2006; 28: 64-68.
30. Turello R, Rentsch K, Di Paolo E et al.: Renal failure after high-dose methotrexate in a child homozygous for MTHFR C677T polymorphism. Pediatric Blood and Cancer 2008; 50: 154-156.

