*Hubert Arasiewicz1, Marta Samborska2, Natalia Salwowska1, Martyna Zbiciak-Nylec2, Ligia Brzezińska-Wcisło1, 2
Hydroxychloroquine – drug characterization and the most frequently observed adverse reactions in the group of patients with diagnosed alopecia cicatricans
Hydroksychlorochinina – charakterystyka leku i najczęściej obserwowane działania niepożądane w grupie pacjentów z łysieniem bliznowaciejącym
1Chair and Department of Dermatology, School of Medicine in Katowice, Medical University of Silesia, Katowice
Head of Chair and Department: Professor Ligia Brzezińska-Wcisło, MD, PhD
2Andrzej Mielęcki Silesian Independent Public Hospital, Medical University of Silesia, Katowice
Director of Hospital: Włodzimierz Dziubdziela, MD, PhD
Streszczenie
Hydroksychlorochina jest lekiem przeciwmalarycznym, powszechnie używanym w terapii reumatoidalnego zapalenia stawów i innych chorób tkanki łącznej. Jej toksyczność oraz liczba interakcji z innymi lekami są relatywnie niewielkie, co pozwala ograniczyć stosowanie steroidoterapii. W leczeniu łysienia bliznowaciejącego poza miejscowymi steroidami lekarze są skłonni do stosowania hydroksychlorochiny.
Celem pracy było ustalenie częstości występowania działań niepożądanych podczas terapii hydroksychlorochiną.
Grupa pacjentów ze zdiagnozowanym łysieniem bliznowaciejącym była analizowana pod kątem występowania działań niepożądanych w trakcie terapii. Oceniano 52 kobiety oraz jednego mężczyznę.
Zgłaszane działania niepożądane (w kolejności malejącej): dolegliwości gastryczne (n = 16), zaburzenia widzenia (n = 9), zaburzenia widzenia kolorów (n = 3), świąd skóry (n = 3), osłabienie siły mięśniowej/bóle mięśniowe (n = 2), zawroty głowy (n = 1), bóle głowy (n = 1), pokrzywka (n = 2) oraz podwyższone enzymy wątrobowe (n = 1).
Podsumowując, hydroksychlorochina jest lekiem o dobrym profilu bezpieczeństwa i jest coraz częściej stosowana przez dermatologów.
Summary
Hydroxychloroquine is an antimalarial drug commonly used in the therapy of rheumatoid arthritis and other diseases of the connective tissue. Its toxicity and number of interactions with other preparations are relatively low, so it easily allows one to limit the use of glycocorticosteroids. In alopecia cicatricans beside topically or systemically applied glycocorticosteroids doctors are inclined to apply hydroxychloroquine.
The aim of this study was to determine frequency of the adverse events during hydroxychloroquine therapy.
A group of patients with diagnosed alopecia cicatricans who were treated with hydroxychloroquine in terms of the observed reactions was analyzed. The studied group consisted of 52 women and one man.
The adverse reactions reported by the patients included (in descending order): increased gastric symptoms (n = 16), vision disturbances (n = 9), discolorations (n = 3), skin pruritus (n = 3), muscle power decrease/muscle pain (n = 2), vertigo (n = 1), headache (n = 1), urticaria (n = 2) and elevated liver enzymes (n = 1). Most adverse reactions were reported within up to three months after the hydroxychloroquine therapy initiation.
To sum up, hydroxychloroquine is a drug with a good safety profile and is increasingly often prescribed by dermatologists.

Introduction
Hydroxychloroquine is an antimalarial drug commonly used in the therapy of rheumatoid arthritis and other diseases of the connective tissue. Since its discovery in 1955, it has found other numerous applications and occupies an important position in the dermatological treatment repertoire. Its toxicity and number of interactions with other preparations are relatively low, so it easily allows one to limit the use of glycocorticosteroids and consequently, inter alia, to reduce the number of secondary infection cases. The exact mechanism of action of this drug has not been fully explained yet, but numerous clinical observations and experimental studies have recorded its antithrombotic, hypoglycemic and hypolipidemic properties (1).
Alopecia cicatricans (fig. 1) is a rare disease characterized by a progressive course and permanent damage to hair follicles. Despite the unambiguous hyperactivity of the immune system, the etiology of the disease still raises many doubts. Due to a significant deterioration of the patients’ quality of life, lack of social acceptance as well as accompanying symptoms such as pruritus, the burning sensation and considerable hypersensitivity of the scalp, it is often necessary to initiate systemic treatment (2). Beside topically or systemically applied glycocorticosteroids, doctors are increasingly inclined to apply non-biological drugs modifying the course of the disease, which include hydroxychloroquine.
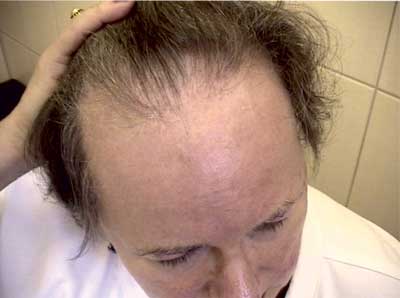
Fig. 1. A female patient with frontal fibrosing alopecia
Pharmacological properties of hydroxychloroquine
Hydroxychloroquine belongs to the group of antimalarial drugs. It is used to treat malaria, rheumatoid arthritis and lupus. It is also applied in dermatology due to its anti-inflammatory properties. Its mechanism of action has not been fully explained yet: it includes, inter alia, influence on lysosomes, phospholipase A2 inhibition, phagocytosis suppression, peroxide synthesis inhibition and intracellular pH increase. This results in a decreased activity of CD4 lymphocytes, inhibition of cytokine release from monocytes and inhibition of antibody production. Due to the observed affinity to pigments, including melanin, the drug may increase the risk of retinopathy development. Moreover, it is applied in cutaneous porphyria because it binds to porphyrins, facilitating their elimination with urine. Literature also mentions aldosterone concentration increase caused by hydroxychloroquine. Its bioavailability is nearly complete, but it should nonetheless be taken during meals to increase its absorption. It reaches the highest serum concentration after approx. 1-3 hours, binding to proteins in approx. 55%. In the case of rheumatoid diseases, the onset of its action may be observed as late as after approx. 4-6 months. It is metabolized mainly in the liver and eliminated mainly via the kidneys. One must exercise caution in pregnant women because the drug penetrates the placental barrier (3-6).
Hydroxychloroquine dosage scheme
The appropriate dosage scheme does not depend on the patient’s actual weight because the preparation deposition in the fatty tissue is scant. It should not exceed 6.5 mg/kg of body weight for so-called ideal weight, even in patients with significant obesity. If the patient’s weight falls within the correct BMI range or is lower than that, the optimal dose of hydroxychloroquine is 5.0 mg/kg of body weight. In most descriptions of dermatological diseases and in the cases of good tolerance, a dose of 400 mg/day was applied. Clinical experience has shown that a bigger dose does not result in the expected improvement and increases the risk of retinopathy development instead. The pharmacokinetic properties of the drug allow one to apply alternative doses in order to optimize the daily dose. This means that one can apply a scheme consisting of alternating 200 mg/day and 400 mg/day doses in order to achieve a daily dose of 300 mg/day. If an adequate response does not follow, one can determine the blood level of hydroxychloroquine, which should equal 750 ng/ml at the initial stage and 500 ng/ml during the administration of the maintenance dose. Given the fact that full saturation with the preparation may take up to several months, one can consider an initial dose of 1,200 mg/day at the beginning of the therapy depending on the clinical situation. Unfortunately, gastric complaints make the abovementioned dosage scheme impossible to apply in most cases. The common view that assumes the influence of smoking on the hydroxychloroquine dosage scheme is rather unclear. Nevertheless, all patients should be educated about the harmful influence of nicotinism, especially if other immunosuppressive drugs are applied. Based on the current findings of experts, it is permissible to administer hydroxychloroquine to pregnant women despite the placental barrier penetration if the therapy brings noticeable results. Dosage modification is related to special clinical situations and aimed at reducing the risk of retinopathy, which is one of the most serious treatment complications. In patients with liver dysfunctions and elderly patients, the drug must be applied with caution because the two factors are characterized by impaired metabolism of the drug, even though they do not increase the risk of retinopathy. Hydroxychloroquine may cause hypoglycemia, which is a very important fact when treating patients who take antidiabetic drugs. In the event of recurring hypoglycemia episodes, the dosage scheme must be reviewed. Special caution must also be exercised when treating patients with kidney dysfunctions (the risk of retinopathy doubles) and women who take tamoxifen (the risk of retinopathy increases five times). Hydroxychloroquine should be avoided in patients with retina dysfunctions, blood picture disturbances, glucose-6-phosphate dehydrogenase deficiency as well as severe stomach and intestine disorders. One must remember that hydroxychloroquine may aggravate the course of psoriasis, porphyria and myasthenia (3-9).
Significant interactions with other medicinal products
Contrary to the common opinion about low toxicity of hydroxychloroquine and the small number of its interactions, one must remember that certain medicinal products constitute a serious threat, while others require particular caution (tab. 1).
Tab. 1. Significant interactions of hydroxychloroquine with other medicinal products
Adalimumab
Alefacept
Anakinra
Anthrax vaccine
Anti-thymocyte globulin
Azathioprine
Live BCG vaccine
Cyclosporin
Digoxin
Diphtheria and tetanus toxoids
Diphtheria and tetanus vaccine/cell-free pertussis vaccine
Inactivated polio vaccine
Etanercept
Golimumab
Hemophilus influenzae type B vaccine
Inactivated hepatitis A vaccine
Hepatitis B vaccine
Human papillomavirus vaccine, bivalent, quadrivalent
Infliximab
Influenza vaccine, quadrivalent, intranasal
Leflunomide
Measles, mumps and rubella vaccine, live
Measles, mumps, rubella and varicella vaccine, live
Mycophenolate
Pneumococcal vaccine, 13-valent, heptavalent
Rabies vaccine
Rilonacept
Rubella vaccine
Sirolimus
Tacrolimus
Temsirolimus
Tocilizumab
Tofacitinib
Typhoid polysaccharide vaccine
Ustekinumab
Varicella virus vaccine, live
Yellow fever vaccine
Shingles vaccine
Astragal
Cholera vaccine
Topically administered dapsone
Denosumab
Echinacea
Mercaptopurine
Methotrexate
Ocrelizumab
Tobramycin |
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
29 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
69 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
129 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 78 zł
Piśmiennictwo
1. Aronson JK: Chloroquine and hydroxychloroquine. [In:] Meyler’s Side Effects of Drugs. The International Encyclopedia of Adverse Drug Reactions and Interactions 2016: 253-267.
2. Otberg N: Primary Cicatricial Alopecias. Dermatol Clin 2013; 31: 155-166.
3. Sanofi-Aventis: Plaquenil product monograph; http://products.sanofi.ca/en/plaquenil.pdf (data dostępu: 19.10.2017).
4. Anthony PF: Updated recommendations on the use of hydroxychloroquine in dermatologic practice. J Am Acad Dermatol 2017; 76: 1176-1182.
5. Rainsford KD, Parke AL, Clifford-Rashotte M et al.: Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 2015; 23(5): 231-269.
6. Jančinová V, Lucová M, Perečko T: Selective inhibition of extracellular oxidants liberated from human neutrophils – A new mechanism potentially involved in the anti-inflammatory activity of hydroxychloroquine. Inter Imphar 2015; 28: 175-181.
7. Fox RI: Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum 1993; 23: 82-91.
8. Fox R: Anti-malarial drugs: possible mechanisms of action in autoimmune disease and prospects for drug development. Lupus 1996; 5 (suppl. 1): S4-10.
9. Costedoat-Chalumeau N, Dunoguè B, Morel N: Hydroxychloroquine: a multifaceted treatment in lupus. Presse Med 2014; 43: e167-180.
10. Furst DE: Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus 1996; 5: S11-15.
11. Schoroeder R: Chloroquine and hydroxychloroquine binding to melanin: some possible consequences for pathologies. Tox Rep 2014; 1: 963-968.
12. Ramser B, Kokot A, Metze D et al.: Hydroxychloroquine modulates metabolic activity and proliferation and induces autophagic cell death of human dermal fibroblasts. J Invest Dermatol 2009; 129: 2419-2426.
13. Lakhanpal S, Ginsburg WW, Michet CL et al.: Eosinophilic fasciitis: clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum 1988; 17: 221-231.
14. Maragh SH, Davis MD, Bruce AJ et al.: Disabling pansclerotic morphea: clinical presentation in two adults. J Am Acad Dermatol 2005; 53: S115-S119.
15. Ben-Zvi I, Kivity S, Langevitz P et al.: Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allerg Immunol 2012; 42: 145-153.
16. Dadhaniyaa N, Sooda I, Patila A et al.: Screening for hydroxychloroquine retinal toxicity: Current recommendations. Apollo Medicine 2017; 14: 27-30.
17. Costedoat N, Dunoguè B, Leroux G et al.: A critical review of the effects of hydroxychloroquine and chloroquine on the eye. Clin Rev Allergy Immunol 2015; 49(3): 317-326.
18. Melles RB, Marmor MF: The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol 2014; 132: 1453-1460.
19. Marmor MF, Kellner U, Lai TYY et al.: Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 2011; 118: 415-422.
20. Wolfe F, Marmor MF: Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arth Care Res 2010; 62: 775-784.
21. Belizna C: Hydroxychloroquine as an anti-thrombotic in antiphospholipid syndrome. Autoimmunity Reviews 2015; 14: 358-362.
22. Pareek A, Chandurkar N, Thomas N et al.: Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: a double blind, randomized comparison with pioglitazone. Curr Med Res Opin 2014; 7: 1257-1266.

